Use the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 in Problem 2.D1) for the
Question:
Use the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 in Problem 2.D1) for the following:
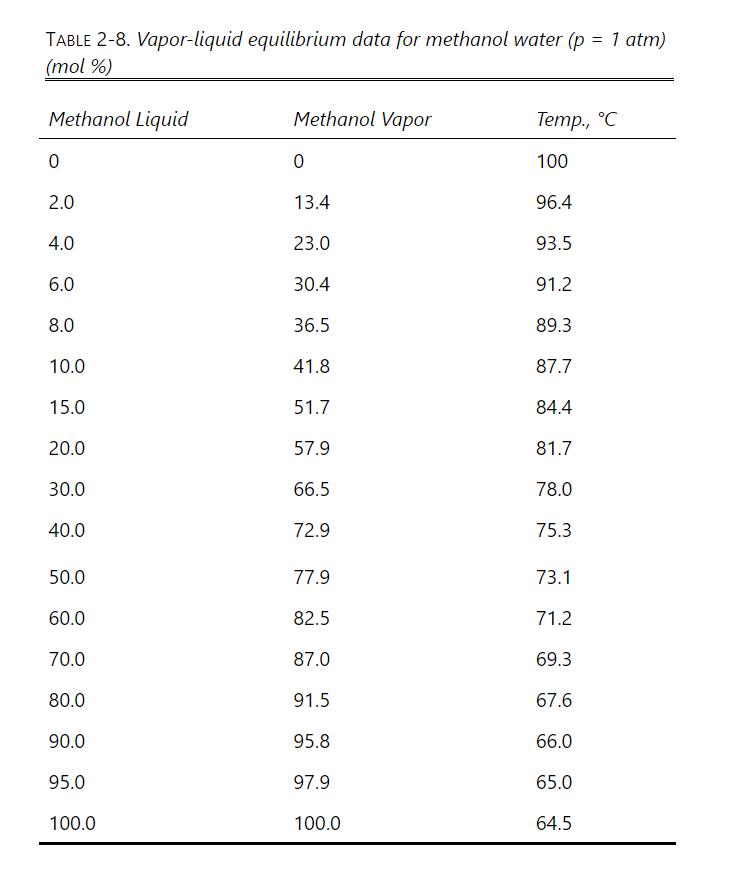
If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction?
Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres?
If water liquid mole fraction is 0.350, what is the water vapor mole fraction?
What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200?
What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





