For the vinyl-chloride process, using heats of formation, heat capacities, and latent heats, carry-out energy balances to
Question:
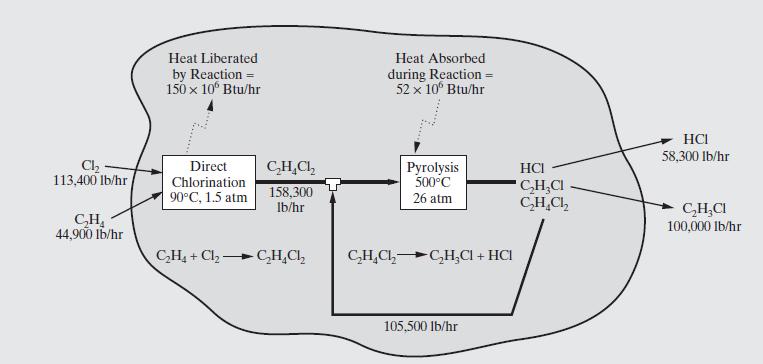
For the vinyl-chloride process, using heats of formation, heat capacities, and latent heats, carry-out energy balances to determine the heats liberated and absorbed in the two reaction operations. Those computed using ASPEN PLUS, as shown in Figure 2.3, are:
(a) Direct chlorination \(=150\) million \(\mathrm{Btu} / \mathrm{hr}\); note the reactants are at \(25^{\circ} \mathrm{C}\) and the effluent stream is at \(90^{\circ} \mathrm{C}\); the pressure is \(1.5 \mathrm{~atm}\).
(b) Pyrolysis \(=52\) million Btu/hr; the reactant is at \(90^{\circ} \mathrm{C}\) and the effluent stream is at \(500^{\circ} \mathrm{C}\); assume the pressure is \(1 \mathrm{~atm}\).
Figure 2.3:-
Step by Step Answer:

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng





