Methyl tertiary butyl ether (MTBE) is used as an antiknock additive in gasoline. It is manufactured by
Question:
Methyl tertiary butyl ether (MTBE) is used as an antiknock additive in gasoline.
It is manufactured by the reaction of isobutene with methanol. The reaction is highly selective and practically any C4 stream containing isobutene can be used as a feedstock:
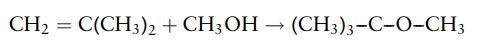
A 10% excess of methanol is used to suppress side reactions. In a typical process, the conversion of isobutene in the reactor stage is 97%. The product is separated from the unreacted methanol and any C4 compounds by distillation.
The essentially pure, liquid, MTBE leaves the base of the distillation column and is sent to storage. The methanol and C4 compounds leave the top of the column as vapor and pass to a column where the methanol is separated by absorption in water. The C4 compounds leave the top of the absorption column, saturated with water, and are used as a fuel gas. The methanol is separated from the water solvent by distillation and recycled to the reactor stage. The water, which leaves the base of the column, is recycled to the absorption column. A purge is taken from the water recycle stream to prevent the buildup of impurities.
1. Draw up a block flow diagram for this process.
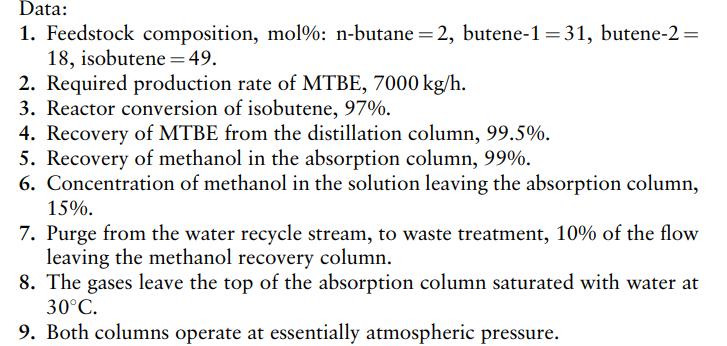
2. Estimate the feeds for each stage.
3. Draw a flowsheet for the process. Treat the C4 compounds, other than isobutene, as one component.
Step by Step Answer:





