(a) A reaction being studied to regenerate oxygen from carbon dioxide on long spaceflights takes place in...
Question:
(a) A reaction being studied to regenerate oxygen from carbon dioxide on long spaceflights takes place in the following two steps: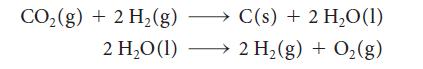
Write the thermochemical equation for the overall reaction.
(b) What enthalpy change would be associated with the production of 32.0 L of oxygen at 0.82 atm and 300 K?
(c) Would the reactor need to be cooled or heated to maintain a constant temperature?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





