(a) Crude petroleum is often contaminated by poisonous hydrogen sulfide gas. The Claus process for the extraction...
Question:
(a) Crude petroleum is often contaminated by poisonous hydrogen sulfide gas. The Claus process for the extraction of sulfur from petroleum has two steps: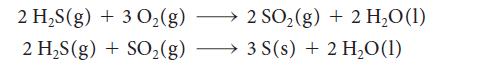
Write a thermochemical equation for the overall reaction that does not contain SO2.
(b) What enthalpy change would be associated with the production of 60.0 kg of sulfur?
(c) Would the reactor need to be cooled or heated to maintain a constant temperature?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





