Question: (a) From the data in Appendix 2A, calculate the enthalpy required to vaporize 1 mol CH 3 OH(l) at 298.2 K. (b) Given that themolar
(a) From the data in Appendix 2A, calculate the enthalpy required to vaporize 1 mol CH3OH(l) at 298.2 K.
(b) Given that the molar heat capacity, CP,m, of liquid methanol is 81.6 J · K–1 · mol–1 and that of gaseous methanol is 43.89 J · K–1 · mol–1, calculate the enthalpy of vaporization of methanol at its boiling point (64.7°C).
(c) Compare the value obtained in part (b) with that found in Table 4C.1. What is the source of difference between these values?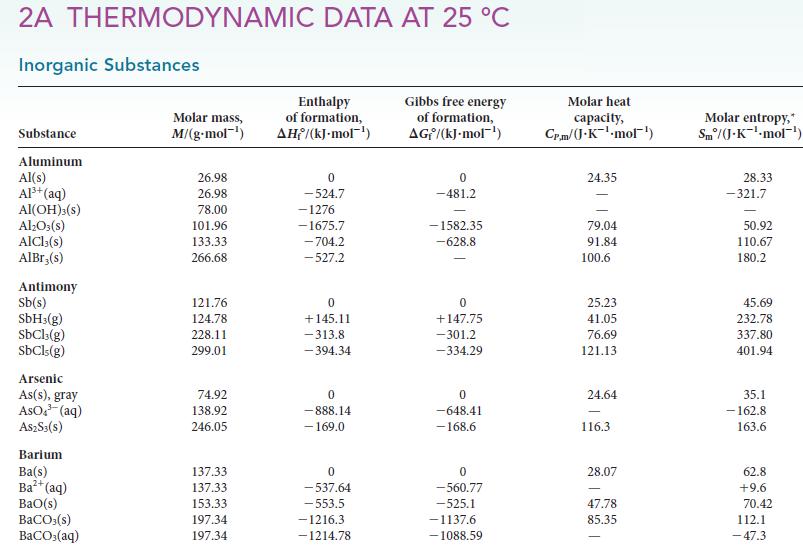
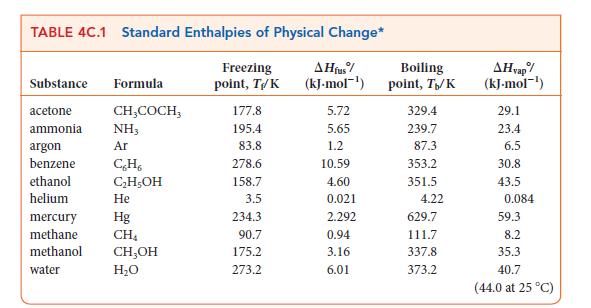
2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s) Al+ (aq) Al(OH)3(S) AlO3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray ASO (aq) A$2S3(S) Barium Ba(s) Ba+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K-mol) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J-K-mol) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
a The enthalpy of vaporization is the heat required for the conversion CH OH 1 will be given by CH3O... View full answer

Get step-by-step solutions from verified subject matter experts


