Question: (a) From the data in Appendix 2A, calculate the enthalpy of vaporization of benzene (C 6 H 6 ) at 298.2 K. The standard enthalpy
(a) From the data in Appendix 2A, calculate the enthalpy of vaporization of benzene (C6H6) at 298.2 K. The standard enthalpy of formation of gaseous benzene is 182.93 kJ · mol–1.
(b) Given that, for liquid benzene, CP,m = 136.1 J · K–1 · mol–1 and that, for gaseous benzene, CP,m = 81.67 J ·K–1 · mol–1, calculate the enthalpy of vaporization of benzene at its boiling point (353.2 K).
(c) Compare the value obtained in part (b) with that found in Table 4C.1. What is the source of the difference between these numbers?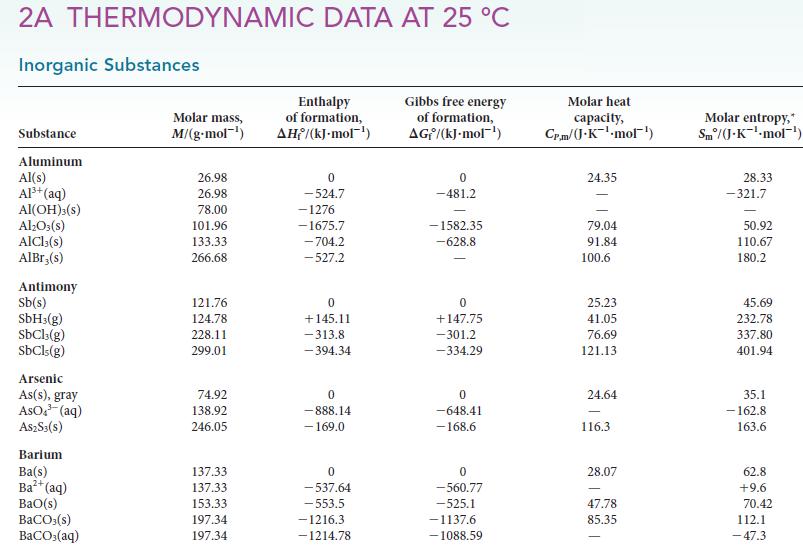
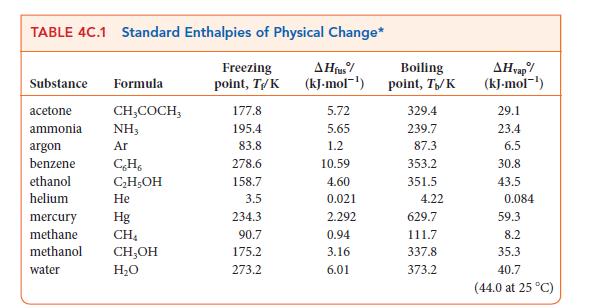
2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s) Al+ (aq) AI(OH)3(s) AlO3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO(aq) A$2S3(S) Barium Ba(s) Ba+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K-mol) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J-K-mol-) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
a 3393 kJ mol 1 b 3094 kJ mol ... View full answer

Get step-by-step solutions from verified subject matter experts


