(a) How many liters of hydrogen at 1.00 atm and 298 K are needed to hydrogenate (i)...
Question:
(a) How many liters of hydrogen at 1.00 atm and 298 K are needed to hydrogenate
(i) 1.00 mol C6H10, cyclohexene; (ii) 1.00 mol C6H6, benzene, completely?
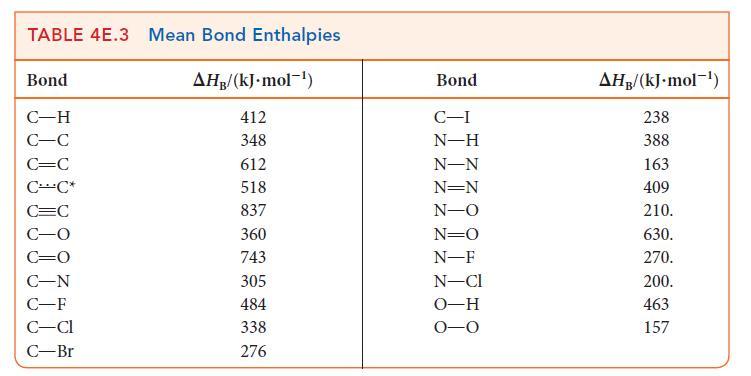
(b) Estimate the reaction enthalpy of each hydrogenation from the average bond enthalpies in Tables 4E.2 and 4E.3.
(c) A Kekulé structure of benzene suggests that its enthalpy of hydrogenation should be three times that of cyclohexene. Do your calculations support this implication? Explain any differences.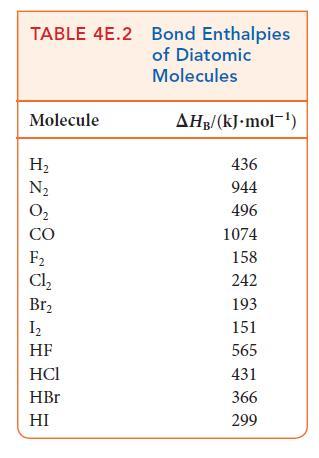
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





