Question: (a) Use a graphing calculator or standard graphing software to make an Arrhenius plot of the data shown here for the decomposition of iodoethane into
(a) Use a graphing calculator or standard graphing software to make an Arrhenius plot of the data shown here for the decomposition of iodoethane into ethene and hydrogen iodide, C2H5I(g) → C2H4 (g) + HI(g), and determine the activation energy for the reaction.
(b) What is the value of the rate constant at 400°C?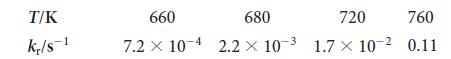
T/K kr/s-1 660 680 720 760 7.2 x 10-4 2.2 x 10- 1.7 x 10-2 0.11
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
To create an Arrhenius plot and determine the activation energy Ea for the reaction you will need to ... View full answer

Get step-by-step solutions from verified subject matter experts


