Question: (a) Use a graphing calculator or standard graphing software to make an Arrhenius plot of the data shown here for the conversion of cyclopropane into
(a) Use a graphing calculator or standard graphing software to make an Arrhenius plot of the data shown here for the conversion of cyclopropane into propene and calculate the activation energy for the reaction.
(b) What is the value of the rate constant at 600°C?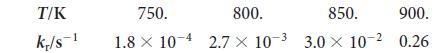
T/K k/s 750. 800. 850. 900. 1.8 104 2.7 x 10-3 3.0 x 10- 0.26
Step by Step Solution
★★★★★
3.42 Rating (152 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

In kr 0 2 4 6 8 1... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


