Question: (a) Use a spreadsheet program or graphing software and data at 1000. K and 1200. K from Table 5G.2 to plot the expression for the
(a) Use a spreadsheet program or graphing software and data at 1000. K and 1200. K from Table 5G.2 to plot the expression for the temperature dependence of ln K given by the dissociation of the diatomic halogens into the atoms, X2 (g) ⇌ 2 X(g).
(b) From the graphs, determine the enthalpies and entropies of dissociation.
(c) Use these data to calculate the standard molar entropies of the gaseous halogen atoms X(g).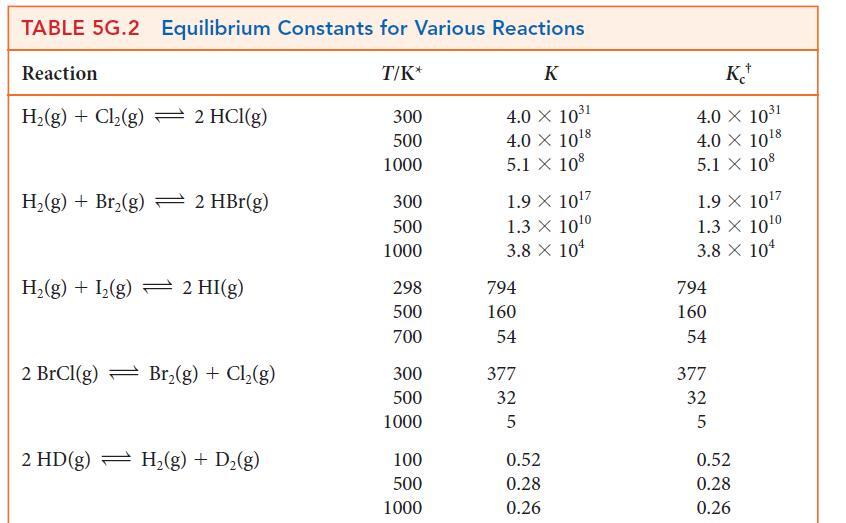
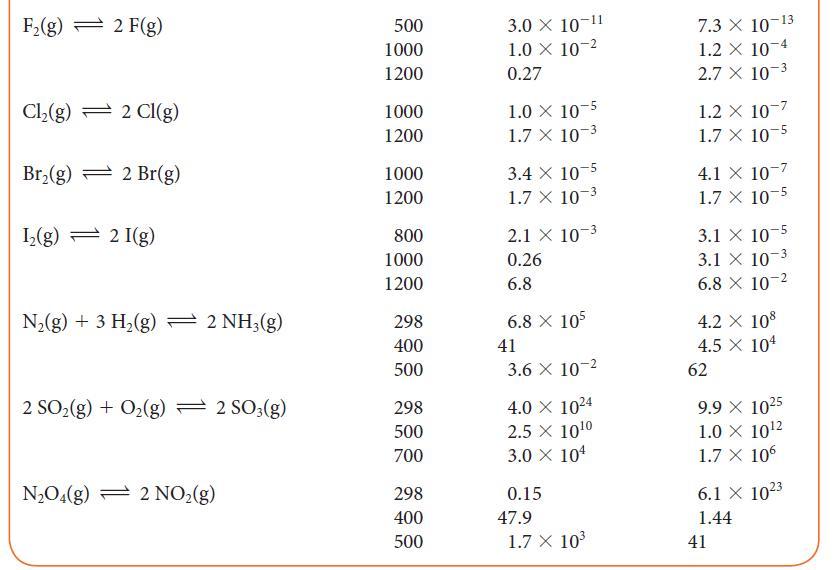
TABLE 5G.2 Equilibrium Constants for Various Reactions T/K* Reaction H(g) + Cl(g) 2 HCl(g) H(g) + Br(g) 2 HBr(g) H(g) + 1(g) 2 HI(g) 2 BrCl(g) Br(g) + Cl(g) 2 HD (g) H(g) + D(g) 300 500 1000 300 500 1000 298 500 700 300 500 1000 100 500 1000 K 4.0 101 4.0 108 5.1 X 108 1.9 X 107 1.3 X 10 3.8 X 104 794 160 54 377 32 5 0.52 0.28 0.26 K 4.0 101 4.0 108 5.1 X 108 1.9 X 107 1.3 100 3.8 X 104 794 160 54 377 32 5 0.52 0.28 0.26
Step by Step Solution
3.29 Rating (164 Votes )
There are 3 Steps involved in it
a Since we are only using two temperatures it really is not necessary to ... View full answer

Get step-by-step solutions from verified subject matter experts


