(a) What is the standard Gibbs free energy of the reaction CO(g) + H 2 O(g) ...
Question:
(a) What is the standard Gibbs free energy of the reaction CO(g) + H2O(g) → CO2(g) + H2(g) when K = 1.00?
(b) From data available in Appendix 2A, estimate the temperature at which K = 1.00.
(c) At this temperature, a cylinder is filled with CO(g) at 10.00 bar, H2O(g) at 10.00 bar, H2(g) at 5.00 bar, and CO2(g) at 5.00 bar. What will be the partial pressure of each of these gases when the system reaches equilibrium?
(d) If the cylinder were filled instead with CO(g) at 6.00 bar, H2O(g) at 4.00 bar, H2(g) at 5.00 bar, and CO2(g) at 10.00 bar, what would the partial pressures be at equilibrium?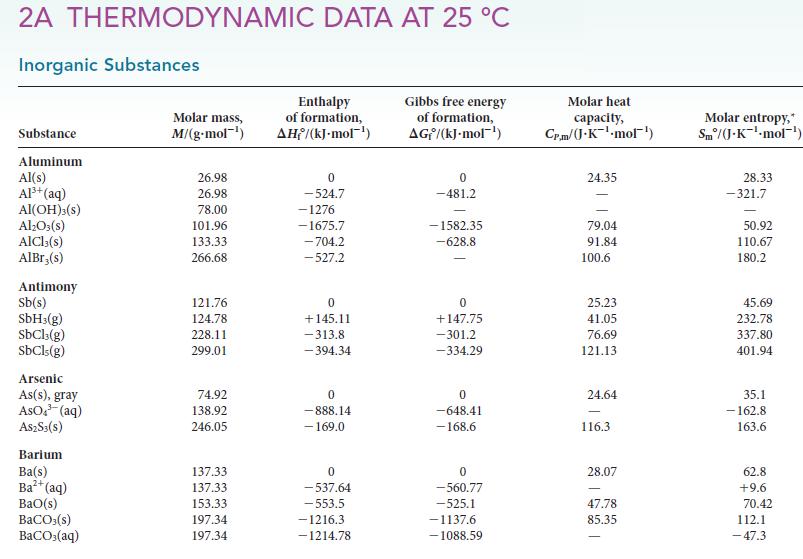
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





