Arrange the following bases in order of increasing strength on the basis of the pK a values
Question:
Arrange the following bases in order of increasing strength on the basis of the pKa values of their conjugate acids, which are given in parentheses:
(a) Aniline (4.63; see Exercise 6C.12);
(b) 2-hydroxyaniline (4.72);
(c) 3-hydroxyaniline (4.17);
(d) 4-hydroxyaniline (5.47). Is there a simple pattern of strengths?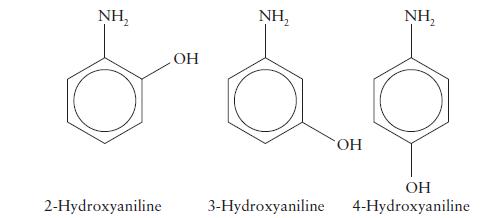
Exercise 6C.12
The value of pKb for aniline is 9.37 and that for 4-chloroaniline is 9.85. Which is the stronger base? Account for the difference in strength.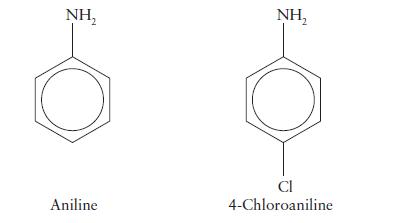
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





