As in Example 6L.1, you are planning to use a Daniell cell to power a model electric
Question:
As in Example 6L.1, you are planning to use a Daniell cell to power a model electric car. However, you find that you do not have standard solutions available. You have only dilute solutions, and you need to know whether they will be adequate to power the car. Calculate the potential of a Daniell cell at 25°C in which the concentration of Zn2+ ions is 0.10 mol · L–1 and that of the Cu2+ ions is 0.0010 mol · L–1.
PLAN Write the balanced equation for the cell reaction and the corresponding expression for Q, and note the value of nr. Then determine Ecell° from the standard potentials in Table 6M.1 or Appendix 2B. Evaluate Q for the stated conditions. Calculate the cell potential by substituting these values into the Nernst equation, Eq. 2b.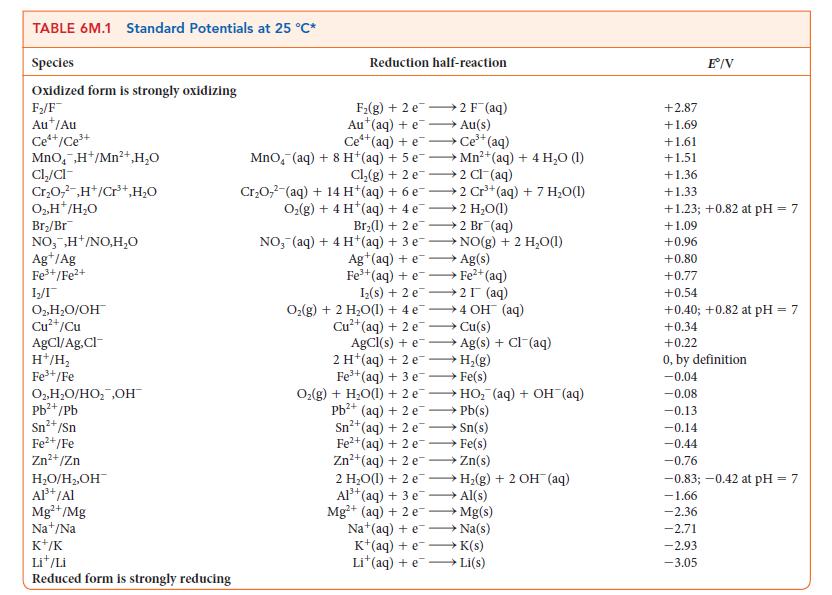
Example 6L.1
For reactions in which the equilibrium constant is very large or very small, it can be difficult to measure the concentration of all species in solution in order to determine K. An alternative method is to measure the cell potential for a reaction and then to use Eq. 1b to obtain the equilibrium constant. Calculate the equilibrium constant at 25.00 °C for the reaction AgCl(s) → Ag+(aq) + Cl–(aq). The equilibrium constant for this reaction is actually the solubility product, Ksp = [Ag+][Cl–], for silver chloride (Topic 6I).
ANTICIPATE Because silver chloride is almost insoluble, you should expect Ksp to be very small (and Ecell° therefore to be negative).
PLAN Follow the procedure in Toolbox 6N.1.
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





