As in Example 7B.1, you are studying the decomposition of N 2 O 5 , but now
Question:
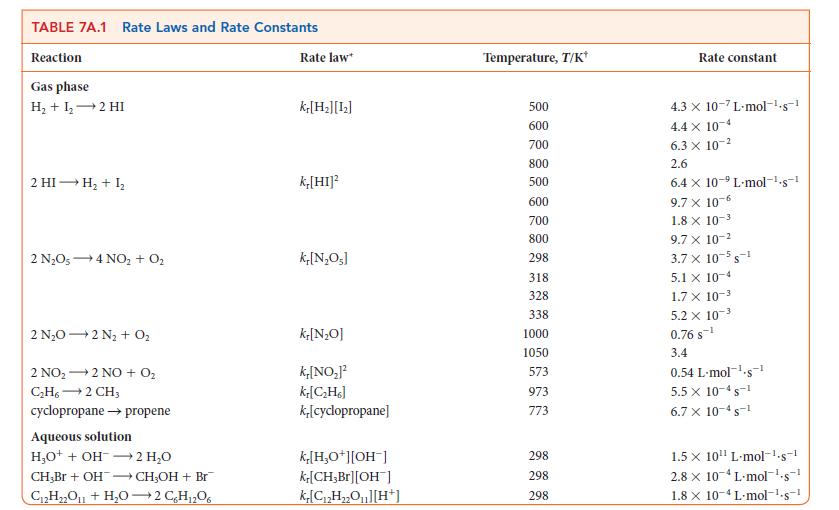
As in Example 7B.1, you are studying the decomposition of N2O5, but now you need to know how long it takes for a given amount of N2O5 to decompose. A sample of N2O5 is allowed to decompose by the reaction 2 N2O5(g) → 4 NO2(g) + O2(g). How long will it take for the concentration of N2O5 to decrease from 20. mmol · L–1 to 2.0 mmol · L–1 at 65°C? Use the data in Table 7A.1.
PLAN First, identify the order of the reaction in N2O5 by referring to Table 7A.1. If the reaction is first order, rearrange Eq. 1a into an equation for t in terms of the given concentrations. Then substitute the numerical value of the rate constant and the concentration data and evaluate t.![[A], [A]o [A] = [A]oe In -k,t -ket (la) (1b)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/4/0/2036590106be56ef1703940203735.jpg)
Example 7B.1
In polar stratospheric clouds, nitrogen can be found as N2O5, which takes part in the ozone cycle that protects life on Earth. However, N2O5 decomposes over time. If you were studying the role of the atmosphere in climate change, you might need to know how much N2O5 remains after a given period of time. What concentration of N2O5 remains 10.0 min (600. s) after the start of its decomposition at 65°C in the reaction 2 N2O5(g) → 4 NO2(g) + O2(g) when its initial concentration was 0.040 mol · L–1? See Table 7A.1 for the rate law.
ANTICIPATE Because the reactant decays with time, you should expect a concentration that is lower than the initial value, but to assess the extent of the change you must do the calculation.
PLAN First identify the order of the reaction. If the reaction is first order in the specified reactant, use the exponential form of the first-order rate law (Eq. 1b) to find the new concentration of the reactant.![]()
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





