Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take
Question:
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in basic solution. Identify the oxidizing agent and reducing agent in each reaction.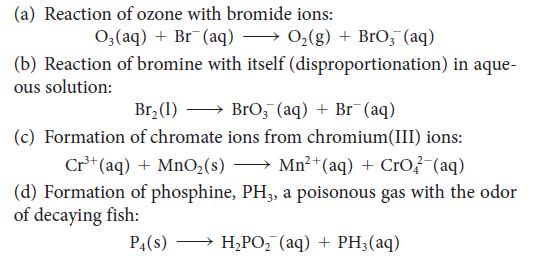
Transcribed Image Text:
(a) Reaction of ozone with bromide ions: O₂ (aq) + Br (aq) → O₂(g) + BrO₂ (aq) (disproportionation) in aque- (b) Reaction of bromine with itself ous solution: Br₂ (1) →BrO₂ (aq)+ Br(aq) (c) Formation of chromate ions from chromium(III) ions: Cr³+ (aq) + MnO₂ (s) Mn²+ (aq) + CrO2 (aq) (d) Formation of phosphine, PH3, a poisonous gas with the odor of decaying fish: P4(S) H₂PO₂ (aq) + PH3 (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 3 03g Braq 3 0g BrO aq is the oxidizing agent and Br is the reducing ...View the full answer

Answered By

CHARLES AMBILA
I am an experienced tutor with more than 7 years of experience. I have helped thousands of students pursue their academic goals. My primary objective as a tutor is to ensure that students have easy time handling their academic tasks.
5.00+
109+ Reviews
323+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in basic solution. Identify the oxidizing agent and reducing agent in...
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
Solve this system of equations -3x-y 5 -3x - 4y 83 || y = 1 11
-
Market-share and market-size variances (continuation of 14-25) Soda-King prepared the budget for 2009 assuming a 10% market share based on total sales in the western region of the United States. The...
-
Auto pistons at Yongpin Zhous plant in Shanghai are produced in a forging process, and the diameter is a critical factor that must be controlled. From sample sizes of 10 pistons produced each day,...
-
How is $t$ test conducted for two independent samples?
-
On January 1, 2019, Parker, Inc., a U.S.-based firm, acquired 100 percent of Suffolk PLC located in Great Britain for consideration paid of 52,000,000 British pounds (), which was equal to fair...
-
Hitzu Company sold a copier (that costs $3,500) for $7,000 cash with a two-year parts warranty to a customer on August 16 of Year 1. Hitzu expects warranty costs to be 4% of dollar sales. It records...
-
Calculate the pH of 8.23 * 10 7 m NaNH 2 (aq)
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A total charge of 4.5 kC...
-
Would a mutation that inactivated the lac repressor and prevented it from binding to the lac operator site result in the constitutive expression of the lac operon under all conditions? Explain. What...
-
5.1. Compore E(2), Elin
-
1. A three stage cross current extraction system is proposed to extract 1000 kg of aqueous solution containing 35 mass % trimethyl amine (TMA) and 65 % water using benzene as a solvent (98 % benzene...
-
To conduct this manufacturing they import certain electronic components from countries like Japan and South Korea. The market for the manufactured tools are the U.S.A, Australia, Canada and United...
-
Information on last year's staffing and turnover is summarized in Table 1. You can assume this pattern will be continue. < +Table 14 Position Executives Sales Staff Management Designers Engineers...
-
A thermistor has resistance of 12 KQ at temperature of 25 C and its constant 3-3750 K is incorporated in the bridge shown. If the input temperature range is (0 to 100) C.The Bridge should be balanced...
-
How could we implement process costing in a plant that has a sequential production process? For example, assume a plant has two separate departmentsparts are fabricated in department 1 and assembled...
-
What are the two components of a company's income tax provision? What does each component represent about a company's income tax provision?
-
In the DebyeHckel theory, the counter charge in a spherical shell of radius r and thickness dr around the central ion of charge +Q is given by Q 2 re r dr. Calculate the radius at which the counter...
-
Calculate the solubility of CaCO 3 (K sp = 3.4 10 -9 ) a. In pure H 2 O. b. In an aqueous solution with I = 0.0250 mol kg 1 . For part (a), do an iterative calculation of and the solubility until...
-
Calculate the probability of finding an ion at a distance greater than 1/ from the central ion.
-
PV of a Combination of a Single Lump Sum and a Series of Equal Sums (annuity): To raise capital for construction of a building for company headquarters, Alberto Company issued $2,000,000, 8%, 10-year...
-
Assuming that the assessed valuation of property within the City of Bingham is $1,409,090,909, and the legal general obligation debt limit is 5 percent of assessed valuation, compute the legal debt...
-
! Required information [The following information applies to the questions displayed below.] Laker Company reported the following January purchases and sales data for its only product. For specific...

Study smarter with the SolutionInn App


