Question: Before checking the numbers, which substance would you expect to have the higher standard molar entropy, CH 3 COOH(l) or CH 3 COOH(aq)? Having made
Before checking the numbers, which substance would you expect to have the higher standard molar entropy, CH3COOH(l) or CH3COOH(aq)? Having made this prediction, examine the values in Appendix 2A and explain your findings.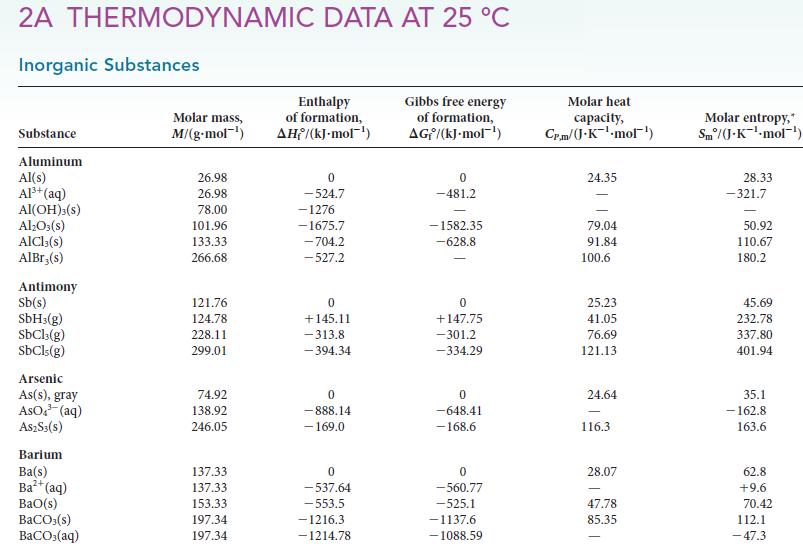
2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s) Al+ (aq) Al(OH)3(S) AlO3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO (aq) A$2S3(S) Barium Ba(s) Ba+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K-mol) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J.K-mol-) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
Firstly let me explain the concept of entropy Entropy often denoted as S is a measure of the number ... View full answer

Get step-by-step solutions from verified subject matter experts


