Three liquid samples with known masses are heated to their boiling points with the use of a
Question:
Three liquid samples with known masses are heated to their boiling points with the use of a heater rated at 500. W. Once their boiling points are reached, heating continues for 4.0 min and some of each sample is vaporized. After 4.0 min, the samples are cooled and the masses of the remaining liquids are determined.
The process is performed at constant pressure.
(a) Using the following data, calculate ΔSvap and ΔHvap for each sample. Assume that all the heat from the heater goes into the sample.
(b) What do the values of ΔSvap indicate about the relative degree of order in the liquids?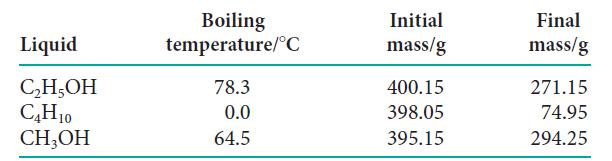
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





