Calculate the standard reaction Gibbs free energy for the following cell reactions: (a) 3 Cr+ (aq) +
Question:
Calculate the standard reaction Gibbs free energy for the following cell reactions: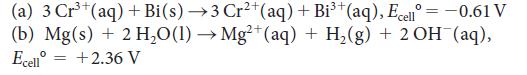
Transcribed Image Text:
(a) 3 Cr³+ (aq) + Bi(s) →3 Cr²+ (aq) + Bi³+ (aq), Ecell = -0.61 V (b) Mg(s) + 2 H₂O(1)→ Mg²+(aq) + H₂(g) + 2 OH (aq), Ecell +2.36 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the standard reaction Gibbs free energy G for a cell reaction we can use the equation G ...View the full answer

Answered By

Deborah Joseph
My experience has a tutor has helped me with learning and relearning. You learn everyday actually and there are changes that are made to the curriculum every time so being a tutor has helped in keeping me updated about the present curriculum and all.
I have also been able to help over 100 students achieve better grades particularly in the categories of Math and Biology both in their internal examinations and external examinations.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict the standard cell potential and calculate the standard reaction Gibbs free energy for galvanic cells having the following cell reactions: (a) 3 Zn(s) + 2 Br (aq) 3 Zn+ (aq) + 2 Bi(s) (b) 2...
-
Calculate the standard reaction Gibbs free energy for the following cell reactions: 4+ (a) 2 Ce (aq) + 3I (aq) 2 Ce(aq) + 13 (aq), Ecell +1.08 V (b) 6 Fe+(aq) +2 Cr+(aq) + 7 HO(1) 6 Fe+ (aq) + CrO2...
-
Suppose that you are in an engineering competition in which commercial power sources are prohibited. You might decide to use a Daniell cell to power a model electric car. You will need to know the...
-
Discuss what you see as the role of ethics as it pertains to management and managers. Does management, in your view, help shape the values and ethics of an organization? What about an organization's...
-
Weighted-average method, assigning costs. Bio Doc Corporation is a biotech company based in Milpitas. It makes a cancer-treatment drug in a single processing department. Direct materials are added at...
-
The Lauren Shur Tub Company manufactures two lines of bathtubs, called model A and model B. Every tub requires blending a certain amount of steel and zinc; the company has available a total of 25,000...
-
Explain the difference between the Address and Addresses classes.
-
Exercise 9- 11 Honoring a note P3 Prepare journal entries to record these selected transactions for Vitalo Company. Nov. 1 Accepted a $ 6,000, 180- day, 8% note dated November 1 from Kelly White in...
-
Klaus is new to agile project management. What key difference should he understand while managing agile projects? Explain
-
Calcium acetate, Ca(CH 3 CO 2 ) 2 (aq), is used to treat patients with a kidney disease that results in high levels of phosphate ions in the blood. The calcium binds to the phosphates so that they...
-
Sometimes the pH must be converted into the hydronium ion concentration. The quickest way to find the hydronium ion concentration in a solution is to use a pH meter to measure the pH and then...
-
An experiment was conducted regarding a quantitative analysis of factors found in high-density lipoprotein (HDL) in a sample of human blood serum. Three variables thought to be predictive of, or...
-
1: Prepare the acquisition analysis as at 1 July 20x3 for the acquisition of B Ltd by A Ltd. Show all calculations. Q2: Prepare the journal entries for these transactions in the records of A Ltd, On...
-
One of your work colleagues has approached you for help with completing a spreadsheet model that they are constructing to determine whether they should sell their existing motor vehicle to NLC and...
-
How to calculate the 5yr returns? BOND Shares National Munt Bond Schwab US TIPS Vanguard Emerg Met Bond Blackrock ESG Bond Shares 1-3 Year Treasury BOND TOTAL MUB SCHF SOMA BIAAX SHY [$117.05]]...
-
For each transaction, indicate in which journal it should be recorded. Sales Journal Cash Receipts Journal Purchases Journal Cash Payments Journal General Journal Returned products to a supplier....
-
During the month of March 2022 the company John Services, Ltd. realized the following transactions March 1, Paid a 12 month insurance policy for $36,000 March 2: Paid the employee $44,000 for their...
-
Why do organizations use both leading and lagging indicators of performance?
-
Perform the operation by first converting the numerator and denominator to scientific notation. Write the answer in scientific notation. 7,200,00/0.000009
-
The probability density for a particle in a box is an oscillatory function even for very large energies. Explain how the classical limit of a constant probability density that is independent of...
-
Explain using words, rather than equations, why if V (x, y, z) V x (x) + V y ( y) + V z (z), the total energy eigenfunctions cannot be written in the form (x, y, z) = X (x)Y( y)Z(z).
-
Can a guitar string be in a superposition of states or is such a superposition only possible for a quantum mechanical system?
-
The below table shows the summary of the Linear Regression model. What is wrong with the model? [1 Points] Row ID Row 3 S Variable D Coeff. DP>|t| views_trailer 0.229 0 Row 1 visitors 0.127 0 Row 21...
-
In O-notation, what is the worst-case running time of the following code as a function of n? Give the best answer you can (the most informative symbol with the simplest function). Here A,] represents...
-
Consider the following set of data points (xi, Yi): X 0 2 1234 1037 3-1 4 0 (a) (10 pts) Set up the system of equations required to compute the spline parameters g" (xi) using natural spline (aka...

Study smarter with the SolutionInn App


