Classify each of the following as a strong acid, weak acid, strong base, or weak base in
Question:
Classify each of the following as a strong acid, weak acid, strong base, or weak base in aqueous solution.
a. \(\mathrm{HNO}_{2}\)
b. \(\mathrm{HNO}_{3}\)
c. \(\mathrm{CH}_{3} \mathrm{NH}_{2}\)
d. \(\mathrm{NaOH}\)
e. \(\mathrm{NH}_{3}\)
f. HF
g. 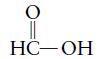
h. \(\mathrm{Ca}(\mathrm{OH})_{2}\)
i. \(\mathrm{H}_{2} \mathrm{SO}_{4}\)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





