Question: Complete a Lewis structure for the compound shown below, then answer the following questions. How many carbon atoms are sp 2 hybridized? How many CON
Complete a Lewis structure for the compound shown below, then answer the following questions. How many carbon atoms are sp2 hybridized? How many CON bonds are formed by the overlap of an sp3 hybridized carbon with an sp3 hybridized nitrogen? How many lone pairs of electrons are in the Lewis structure of your molecule? How many π bonds are present?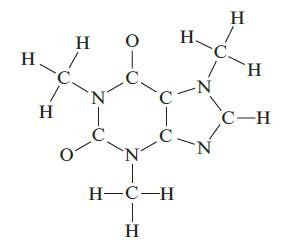
H H H N T O 0- C N H-C-H T H H. C. -C -N N H H C-H
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Answer There are four carbon atoms sp2 hybridized There are three CON bonds formed by ... View full answer

Get step-by-step solutions from verified subject matter experts


