Question: Consider the question posed in Exercise 6N.19 except that a saturated calomel electrode (the solution is saturated with KCl instead of having [Cl ]
Consider the question posed in Exercise 6N.19 except that a saturated calomel electrode (the solution is saturated with KCl instead of having [Cl–] = 1.00 mol · L–1) is used in place of the standard calomel electrode. How will this replacement change your answers to Exercise 6N.19? The solubility of KCl is 35 g · (100 mL H2O)–1.
Exercise 6N.19
Suppose the reference electrode for Table 6M.1 were the standard calomel electrode, Hg2Cl2/Hg, Cl2([Cl2] = 1.00 mol · L–1), with its E° set equal to 0. Under this system, what would be the potential for
(a) The standard hydrogen electrode;
(b) The standard Cu2+/Cu redox couple?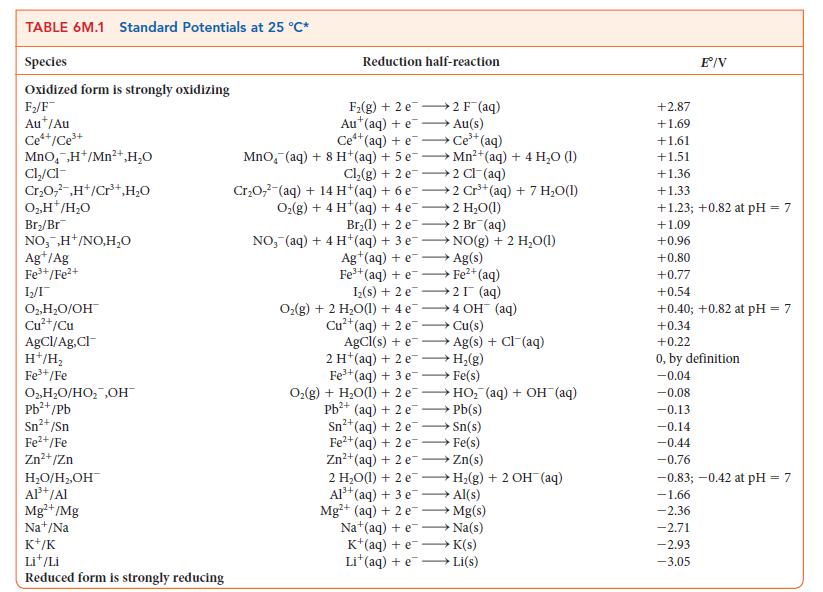
TABLE 6M.1 Standard Potentials at 25 C* Species Oxidized form is strongly oxidizing F/F Aut/Au Ce*+/Ce+ MnO,H+/Mn+ HO Cl/cl- CrO,,H+/Cr+,HO 0.H/HO Br/Br NO,,H+/NO,HO Ag+/Ag Fe+/Fe+ 12/1 O2, HO/OH Cu+/Cu AgCl/Ag,Cl- H+/H Fe+/Fe O,HO/HO ,OH Pb+/Pb Sn+ /Sn Fe+/Fe Zn+/Zn HO/H,OH Al+ /Al Mg+/Mg Na+/Na K+/K Lit/Li Reduced form is strongly reducing Reduction half-reaction F(g) +2 e Au (aq) + e 2 F (aq) Au(s) Ce+ (aq) + e Ce+ (aq) MnO, (aq) + 8 H+(aq) + 5 eMn+(aq) + 4 HO (1) Cl(g) + 2 e 2 Cl(aq) CrO7 (aq) + 14 H(aq) + 6 e2 Cr+(aq) + 7 HO(1) O(g) + 4 H (aq) + 4e 2 HO(1) Br(1) + 2 e2 Br (aq) NO, (aq) + 4 H+(aq) + 3 e Ag+ (aq) + e NO(g) + 2 HO(1) Ag(s) Fe+(aq) +e Fe+ (aq) Iz(s) + 2 e 2I (aq) O(g) + 2 HO(1) + 4e4 OH (aq) Cu(s) 2+ Cu+(aq) + 2 e AgCl(s) + e 2 H (aq) + 2 e Fe+(aq) + 3 e Ag(s) + Cl (aq) H(g) Fe(s) O(g) + HO(1) + 2 eHO (aq) + OH(aq) Pb+ (aq) + 2 e Pb(s) Sn+ (aq) + 2 e Sn(s) Fe+ (aq) + 2 e Fe(s) Zn+ (aq) + 2 eZn(s) 2 HO(1) + 2 eH(g) + 2 OH (aq) Al+ (aq) + 3 e- Al(s) Mg+ (aq) + 2 e Mg(s) Na (aq) + e Na(s) K+ (aq) + eK(s) Lit(aq) +eLi(s) +2.87 +1.69 +1.61 +1.51 E/V +1.36 +1.33 +1.23; +0.82 at pH = 7 +1.09 +0.96 +0.80 +0.77 +0.54 +0.40; +0.82 at pH = 7 +0.34 +0.22 0, by definition -0.04 -0.08 -0.13 -0.14 -0.44 -0.76 -0.83; -0.42 at pH = 7 -1.66 -2.36 -2.71 -2.93 -3.05
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
In Exercise 6N19 the standard calomel electrode Hg2C12Hg C12C12 100 mol L 1 is used as the reference ... View full answer

Get step-by-step solutions from verified subject matter experts


