Question: Consider your Lewis structure for the computer-generated model of caffeine shown in Exercise 108 of Chapter 13. How many (mathrm{C}) and (mathrm{N}) atoms are (s
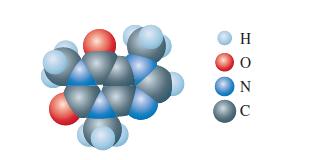
Consider your Lewis structure for the computer-generated model of caffeine shown in Exercise 108 of Chapter 13. How many \(\mathrm{C}\) and \(\mathrm{N}\) atoms are \(s p^{2}\) hybridized in your Lewis structure for caffeine? How many \(\mathrm{C}\) and \(\mathrm{N}\) atoms are \(s p^{3}\) hybridized? \(s p\) hybridized? How many \(\sigma\) and \(\pi\) bonds are in your Lewis structure?
Lewis structure of Exercise 108 of Chapter 13
ON O H
Step by Step Solution
3.47 Rating (147 Votes )
There are 3 Steps involved in it
The caffeine Lewis structure shows that there are four nitrogen N atoms and nine carbon C atoms N at... View full answer

Get step-by-step solutions from verified subject matter experts


