Cyanomethane, commonly known as acetonitrile, CH 3 CN, is a toxic volatile liquid which is used as
Question:
Cyanomethane, commonly known as acetonitrile, CH3CN, is a toxic volatile liquid which is used as a solvent to purify steroids and to extract fatty acids from fish oils. Acetonitrile can be synthesized from methyl isonitrile by the isomerization reaction CH3NC(g) → CH3CN(g).
(a) Draw the Lewis structures of methyl isonitrile and cyanomethane; assign a hybridization scheme to each C atom and indicate whether each molecule is polar or nonpolar.
(b) Estimate ΔH° for the isomerization reaction by using the mean bond enthalpies in Table 4E.3. Which isomer has the lower enthalpy of formation?
(c) The isomerization reaction obeys the first-order rate law, Rate = kr[CH3NC], in the presence of argon. The activation energy for this reaction is 161 kJ · mol–1, and the rate constant at 500. K is 6.6 * 10–4 s–1. Calculate the rate constant at 300. K and the time (in seconds) needed for the concentration of CH3NC to decrease to 75% of its initial value at 300. K.
(d) Draw a reaction profile for the isomerization reaction.
(e) Calculate the temperature at which the concentration of CH3NC will decrease to 75% of its original concentration in 1.0 h.
(f) What purpose does the excess argon serve?
(g) At low concentrations of Ar, the rate is no longer first order in CH3NC; suggest an explanation.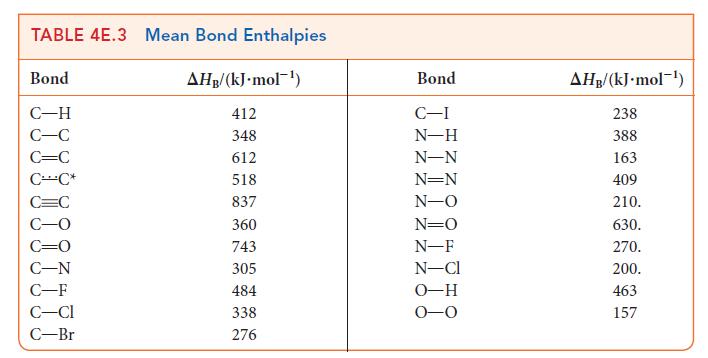
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





