Question: As the principal quantum number n increases, the electron is more likely to be found far from the nucleus. It can be shown that for
As the principal quantum number n increases, the electron is more likely to be found far from the nucleus. It can be shown that for H and for ions with only one electron such as He+,
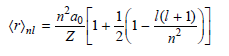
Calculate the value of n for an s state in the hydrogen atom such that Ωrλ= 500a0. Round up to the nearest integer. What is the ionization energy of the H atom in this state in electron-volts? Compare your answer with the ionization energy of the H atom in the ground state.
1(1 + 1) n'ao (r)ml 1. n-
Step by Step Solution
3.45 Rating (174 Votes )
There are 3 Steps involved in it
For the ground state I 136057 eV and for n 19 ... View full answer

Get step-by-step solutions from verified subject matter experts


