Question: EDTA is used as a complex agent in chemical analysis. Solutions of EDTA, usually containing the disodium salt Na 2 H 2 EDTA, are also
EDTA is used as a complex agent in chemical analysis. Solutions of EDTA, usually containing the disodium salt Na2H2EDTA, are also used to treat heavy metal poisoning. The equilibrium constant for the following reaction is 6.7 × 10-1: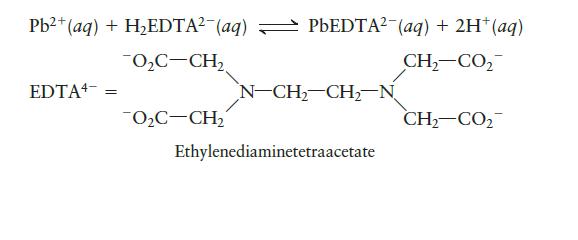
Pb+ (aq) + HEDTA (aq) PbEDTA (aq) + 2H+ (aq) 0C-CH CH,CO, CH,CO2 EDTA = OC-CH = N-CH-CH-N Ethylenediaminetetraacetate
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


