Evaluate K c for each of the following equilibria from the value of K: (a) 2 SO(g)
Question:
Evaluate Kc for each of the following equilibria from the value of K: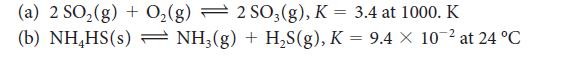
Transcribed Image Text:
(a) 2 SO₂(g) + O₂(g) 2 SO3(g), K = 3.4 at 1000. K (b) NH₂HS(s) - NH3(g) + H₂S(g), K = 9.4 x 10-² at 24 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Please note that the concentrations should be in molarity molL molL when substituting t...View the full answer

Answered By

Ajeet Singh
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life.
I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge.
I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields.
Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a teacher. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
4.90+
7+ Reviews
15+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Evaluate K c for each of the following equilibria from the value of K: (a) 2 NOCI(g) (b) CaCO3(s) 2 NO(g) + Cl(g), K = 1.8 10- at 500 K CaO(s) + CO(g), K = 167 at 1073 K
-
Write equilibrium-constant expressions Kc for each of the following reactions. a. N203(g)NO2(8) +NO(g 2NO(g)+O2(g) 2NO2(g) PC13(g) + 3NH3(g)-P(NH2)3(g) + 3HC(g) c. d.
-
Write equilibrium-constant expressions Kc for each of the following reactions. a. N2(g) 2H2g) N2H4(g) b. 2NOCI(g) 2N0(g) + Cl2(g) c. 2NO(g) 2H2(g)N2(8) 2H20(g)
-
At the beginning of the current tennis season, on April 1, 2024, Kicked-Back Tennis Shops inventory consisted of 50 tennis racquets at a cost of $40 each. Kicked-Back uses a perpetual inventory...
-
The separation of isopentane from n-pentane by distillation is difficult (approximately 100 trays are required), but is commonly practiced in industry. Using the extended Antoine vapor pressure...
-
1. Which people would you interview first and why? 2. What type of information would you seek? 3. How would you approach each of the different individuals? 4. Due to Jamess frail condition, how would...
-
A model for chromatographic separation: a case-study problem. An important application of Taylor dispersion is in chromatography. Here pulses of a mixture of solutes are introduced into one end of a...
-
Dorsey Company manufactures three products from a common input in a joint processing operation. Joint processing costs up to the split-off point total $350,000 per quarter. For financial reporting...
-
Review the following scenario: You work in customer service for the local bus station. A bus is running over an hour late due to mechanical issues. Customers are getting very angry and complaining...
-
How would the legal realists (e.g., Karl Llewellyn and Oliver Wendell Holmes) view the current relationship between social psychology and the law?
-
Calculate (a) The molality of 13.63 g of sucrose, C 12 H 22 O 11 , dissolved in 612 mL of water; (b) The molality of CsCl in a 10.00% by mass aqueous solution; (c) The molality of acetone in an...
-
Use data from Table 4C.1 to calculate the vapor pressure of mercury at 275 K. TABLE 4C.1 Standard Enthalpies of Physical Change* Freezing AH fus% (kJ. mol) point, T/K Substance Formula acetone...
-
A partial adjusted trial balance of Gehring Company at January 31, 2014, shows the following. Instructions Answer the following questions, assuming the year begins January 1. (a) If the amount in...
-
Determine which algorithm Minchow is likely to use for the Dynopax sell order. Justify your response. Minchow is also tasked to help EEP exit from a large position in a widely-traded blue chip stock....
-
In a quarter, an investment managers upside capture is 75% and downside capture is 125%. We can conclude that the manager underperforms the benchmark: A. only when the benchmark return is positive....
-
Calculate the market-adjusted cost of the trade. Discuss the finding. Although focused on long-term value, North Circle Advisors will exploit temporary mispricings to open positions. For example,...
-
Which of the following types of style analysis use(s) a bottom-up approach to estimate the risk exposures in a portfolio? A. Returns-based style analysis only B. Holdings-based style analysis only C....
-
An advantage of a returns-based style analysis is that such analysis: A. is comparable across managers. B. is suitable for portfolios that contain illiquid securities. C. can effectively profile a...
-
What is the present value of $1,000, received four years from now, using a periodic interest rate of 7.5% compounded annually?
-
10m solution. If Ka(HA) = 10 then pOH of solution will be [Given : log4=0.6] (A) 6.7 (B) Greater than 6.7 & less than 7.0 (C) Greater 7.0 & less than 7.3 (D) Greater than 7.3
-
Draw all resonance structures for each of the following radicals: (a) (b) (c) (d) (e)
-
Why is the standard state of fugacity, f , equal to the standard state of pressure, P?
-
A compressed cylinder of gas contains 2.74 10 3 g of N 2 gas at a pressure of 3.75 10 7 Pa and a temperature of 18.7C. What volume of gas has been released into the atmosphere if the final pressure...
-
1. By using cofactors Ci; of 10 - -1 A 3 1 0 -42 2 find adjA. Verify that the adj A you obtained is correct by multiplying it with A. 2. For the following matrix 1 -1 1 A = = 1 1 1 1 1 = (3+2 5...
-
Define the function f : R \ {0} R by f(x) = -- (a) Sketch the graph of y = f(x). (b) Find the range of f. (c) Determine whether or not f is bijective. (d) Define a function g: R\{0,1}R by g(x) =...
-
If T:Vw is a linear transformation Show that T is ohe-one it ker T={0}.

Study smarter with the SolutionInn App


