For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or
Question:
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: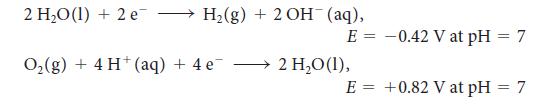 The anode of an electrolytic cell was constructed from (a) Cr; (b) Pt; (c) Cu; (d) Ni. For each case, determine whether oxidation of the electrode or of water will take place at the anode when the electrolyte consists of a 1.0 m solution of the oxidized metal ions at pH = 7.
The anode of an electrolytic cell was constructed from (a) Cr; (b) Pt; (c) Cu; (d) Ni. For each case, determine whether oxidation of the electrode or of water will take place at the anode when the electrolyte consists of a 1.0 m solution of the oxidized metal ions at pH = 7.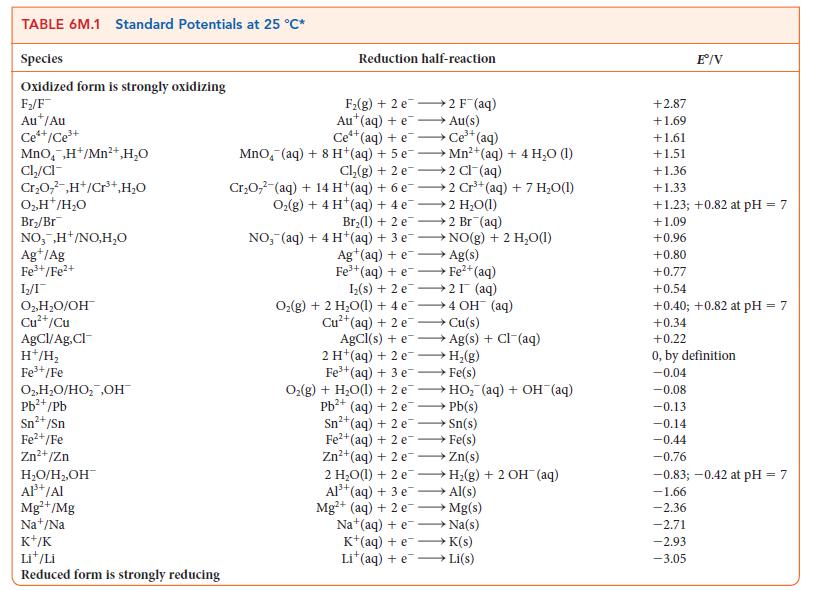
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





