Question: For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: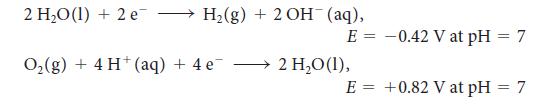 Copper from 200.0 mL of a solution of copper(II) sulfate is plated on to the cathode of an electrolytic cell.
Copper from 200.0 mL of a solution of copper(II) sulfate is plated on to the cathode of an electrolytic cell.
(a) Hydronium ions are generated at one of the electrodes. Is this electrode the anode or cathode?
(b) How many moles of H3O+ are generated if a current of 0.120 A is applied to the cell for 30.0 h?
(c) If the pH of the solution was initially 7.0, what will be the pH of the solution after the electrolysis? Assume no change in the volume of solution.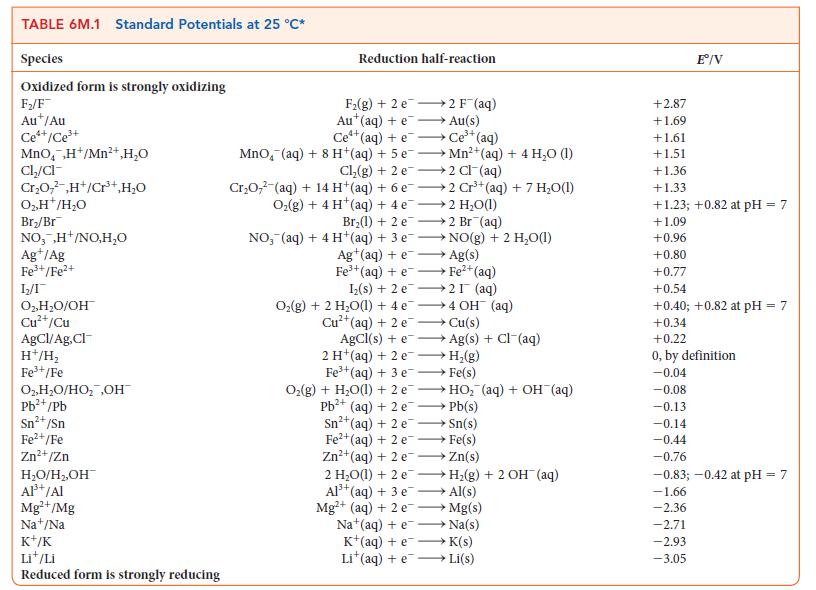
2 HO(1) + 2 e H(g) + 2 OH (aq), E = O(g) + 4 H (aq) + 4e 2 HO (1), E = -0.42 V at pH = 7 +0.82 V at pH = 7
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
a Ano... View full answer

Get step-by-step solutions from verified subject matter experts


