If you are working in a research laboratory preparing a series of compounds, you might be faced
Question:
If you are working in a research laboratory preparing a series of compounds, you might be faced with having to make a choice between acids based on their strengths. Can you decide which to use based only on their formulas? Predict from their molecular structures which acid in each of the following pairs is the stronger:
(a) H2S and H2Se;
(b) H2SO4 and H2SO3;
(c) H2SO4 and H3PO4.
PLAN Identify the relevant trends in the summary in TABLE 6C.7.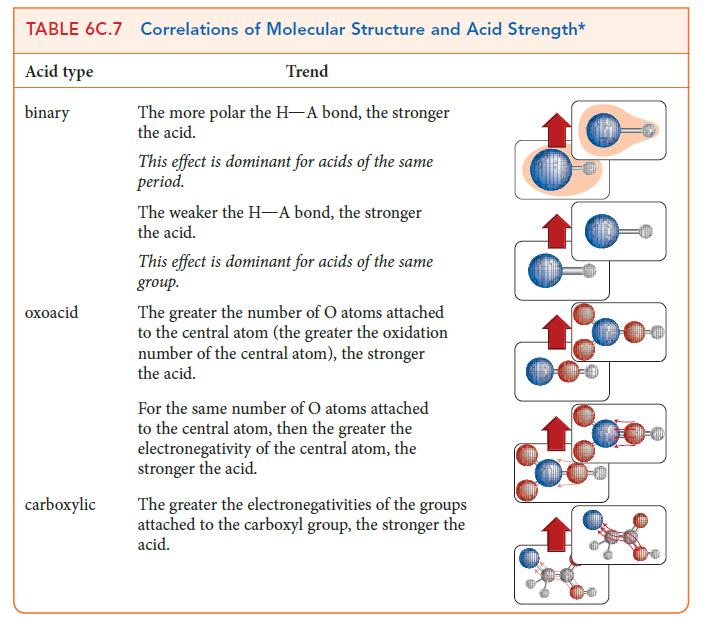
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





