In a gas-phase equilibrium mixture of H 2 , Cl 2 , and HCl at 1000. K,
Question:
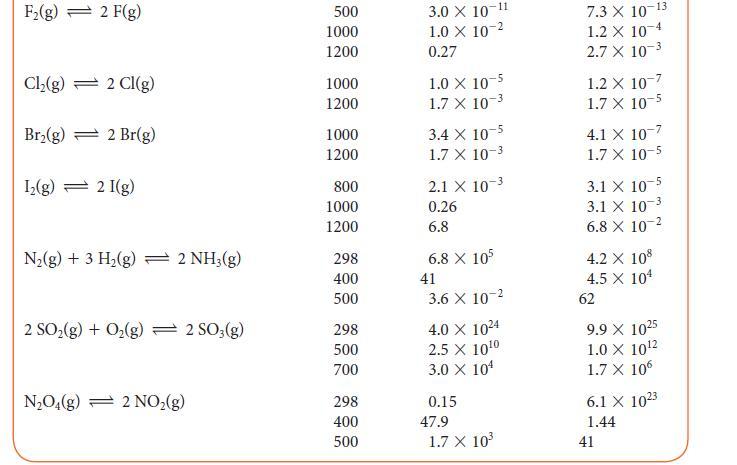
In a gas-phase equilibrium mixture of H2, Cl2, and HCl at 1000. K, [HCl] = 0.352 mmol · L–1 and [Cl2] = 7.21 mmol · L–1.
Use the information in Table 5G.2 to calculate the equilibrium molar concentration of H2.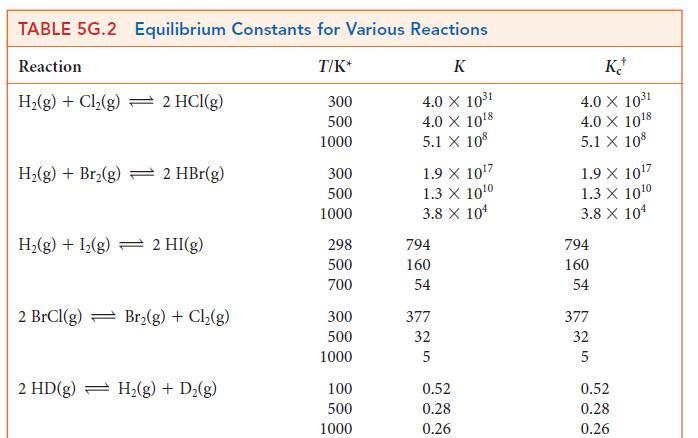
Transcribed Image Text:
TABLE 5G.2 Equilibrium Constants for Various Reactions Reaction T/K* K 4.0 X 10³1 4.0 X 10¹8 5.1 X 108 H₂(g) + Cl₂(g) 2 HCl(g) H₂(g) + Br₂(g) 2 HBr(g) H₂(g) + I₂(g) 2 HI(g) 2 BrCl(g) Br₂(g) + Cl₂(g) 2 HD(g) H₂(g) + D₂(g) 300 500 1000 300 500 1000 298 500 700 300 500 1000 100 500 1000 1.9 X 10¹7 1.3 X 10¹0 3.8 X 10¹ 794 160 54 377 32 5 0.52 0.28 0.26 KJ 4.0 X 10³1 4.0 X 10¹8 5.1 X 108 1.9 X 10¹7 1.3 X 10¹0 3.8 X 104 794 160 54 377 32 5 0.52 0.28 0.26
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The equilibrium constant Kc for the reaction H2g Cl2g 2 HClg at 1000 ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The table below presents equilibrium distribution data for four gaseous solutes dissolved in water, using air as the carrier gas: a. Using a spreadsheet to perform the calculations, prepare a graph...
-
Use the information from Table 2 to estimate the mean and the standard deviation of the ORTOT. Use two decimal points for all of your calculations. Present your network diagram and the earliest and...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Staircase Equipment Company uses a job order cost system. The following data summarize the operations related to production for April 2014, the first month of operations: Materials purchased on...
-
In a music CD warehouse, sometimes orders become deadlocked because two different orders want the last two different CDs. For example, two people might simultaneously order More Themes for Young...
-
A local service station is open 7 days per week, 365 days per year. Sales of 10W40 grade premium oil average 20 cans per day. Inventory holding costs are $0.50 per can per year. Ordering costs are...
-
Identify the four components of a use case and how they affect each other.
-
Endnote Enterprises entered into the following transactions during 2015: 1. Sold merchandise for $52,000 in cash. 2. Purchased a parcel of land. The company paid $12,000 in cash and issued a $30,000...
-
This week we listened the NPR Podcast Hard Work is Irrelevant that discusses the HR structure of Netflix. If you missed the podcast, you may listen to it below. Once you have listened to the entire...
-
What is the vapor pressure of the solvent in each of the following solutions: (a) The mole fraction of glucose is 0.0316 in an aqueous solution at 40C; (b) An aqueous solution at 23C is 0.0240 m...
-
Calculate the vapor pressure of the solvent in each of the following solutions. Use Table 5A.2 to find the vapor pressure of water in (a) An aqueous solution at 100 C in which the mole fraction of...
-
Name reasons for the development of wireless ATM. What is one of the main differences to Internet technologies from this point of view? Why did WATM not succeed as stand-alone technology, what parts...
-
Air is contained within a cylinder in which heat is being transferred to it via a heating coil. Initially the air is at a temperature T and is heated until a final temperature of T2. The total heat...
-
Use the Pythagorean Theorem to determine the value of x and the measurements of all sides of the right triangle Solve for x 17x 17x-1 (Use a comma to separate answers as needed.) OA. For the smaller...
-
Using the balance sheet provided for American Imports, determine the weighted average cost of capital. The firm's tax rate is 40%, the preferred stock pays a dividend of $0.45 per share, the beta of...
-
Determine the force due to hydrostatic pressure on the flat vertical side of a tank which has the shape in feet of the boundaries y = 0, y = 4x-7, y=-4c-7 and the line y = 21. Note that water has...
-
Peter and Gamora have calculated their taxable income to be $188,000 for 2022, which includes $25,000 of net long-term capital gains. They also made $27,000 of estimated payments for 2022. What is...
-
Two divisions with exactly the same return on investment (ROI) can have different residual incomes (RI). Why?
-
Heineken N.V., a global brewer based in the Netherlands, reports the following balance sheet accounts for the year ended December 31, 2016 (euros in millions). Prepare the balance sheet for this...
-
What makes the z direction special such that l 2 , H, and l z commute, whereas l 2 , H, and l x do not commute?
-
How are the spherical harmonics combined to form real p and d functions? What is the advantage in doing so?
-
The zero point energy of the particle in the box goes to zero as the length of the box approaches infinity. What is the appropriate analogue for the quantum harmonic oscillator?
-
A flask containing n moles of helium is being held at a temperature T Kelvins. If the volume of the flask is V liters, the pressure that the nRT V gas exerts on the flask, in atmospheres, is given by...
-
What New techniques in the production of computers make it possible to produce them at a lower cost. What do you think would happen in the market for computers?
-
Are the two lines below parallel, perpendicular, or neither? Justify your answer. 2???? + 3???? = 5 and 6???? + 9???? = 1 Construct two equations for two lines that are NEITHER parallel nor...

Study smarter with the SolutionInn App


