One of the most important industrial reactions is the conversion of nitrogen to ammonia. Because the efficiency
Question:
One of the most important industrial reactions is the conversion of nitrogen to ammonia. Because the efficiency of this reaction governs the economies of nations, it is necessary for chemical engineers to understand its thermodynamic properties. Calculate the standard reaction entropy of N2(g) + 3 H2(g) → 2 NH3(g) at 25°C.
ANTICIPATE You should expect a decrease in entropy, because there is a net reduction in the amount of gas molecules.
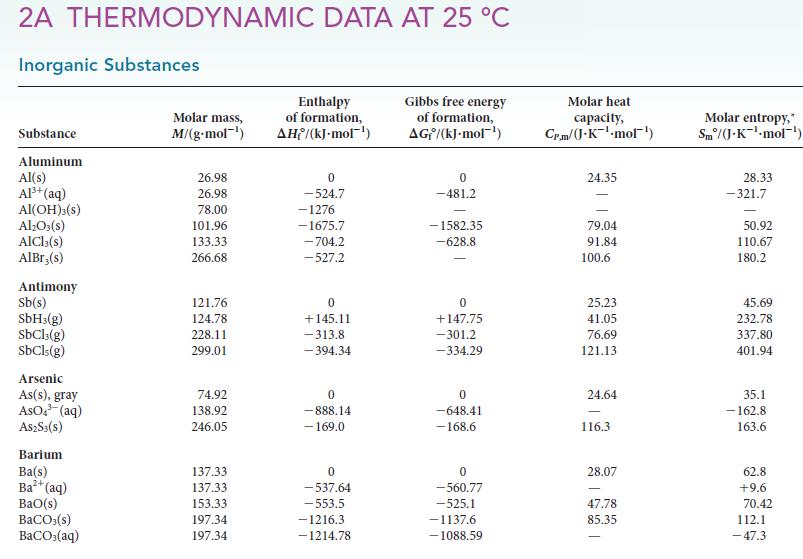
PLAN Use the chemical equation to write an expression for ΔS°, as shown in Eq. 2, and then substitute values from Table 4H.1 or Appendix 2A.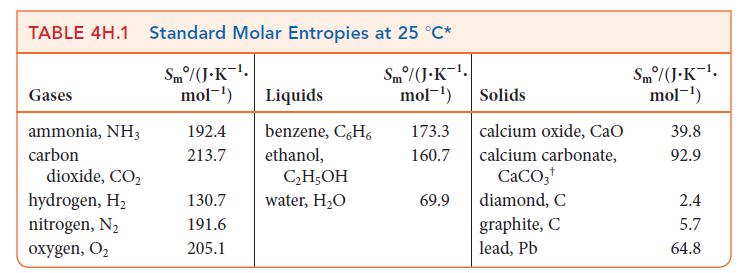
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





