One step in the manufacture of sulfuric acid is the formation of sulfur trioxide by the combustion
Question:
One step in the manufacture of sulfuric acid is the formation of sulfur trioxide by the combustion of SO2 with O2 in the presence of a vanadium(V) oxide catalyst. Suppose you are working out how to increase the equilibrium composition of sulfur trioxide. Predict how the equilibrium composition for the sulfur trioxide synthesis will tend to change when the temperature is raised.
ANTICIPATE Combustion reactions are all exothermic, so you should expect the equilibrium of this to shift in favor of reactants when the temperature is raised.
PLAN Verify that the reaction is exothermic. To find the standard reaction enthalpy, use the standard enthalpies of formation given in Appendix 2A.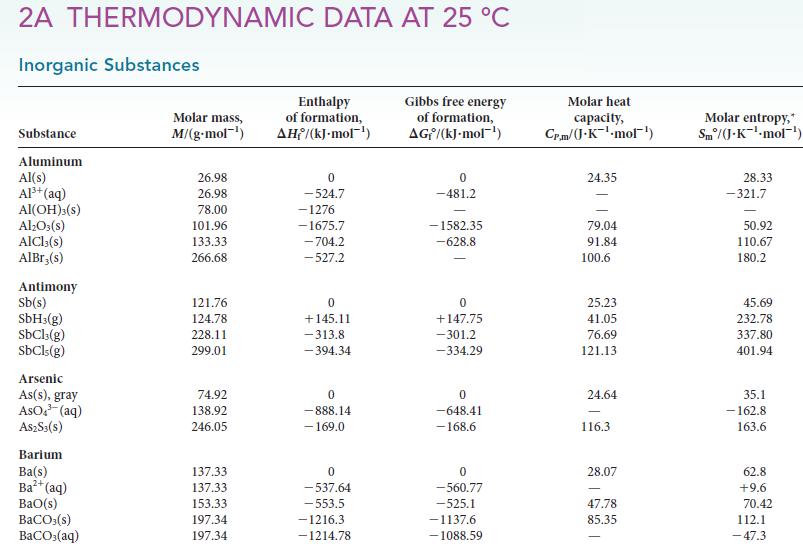
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





