Use the vapor-pressure curve in Fig. 5A.3 to estimate the boiling point of benzene when the atmospheric
Question:
Use the vapor-pressure curve in Fig. 5A.3 to estimate the boiling point of benzene when the atmospheric pressure is
(a) 50. kPa;
(b) 80. kPa.
FIGURE 5A.3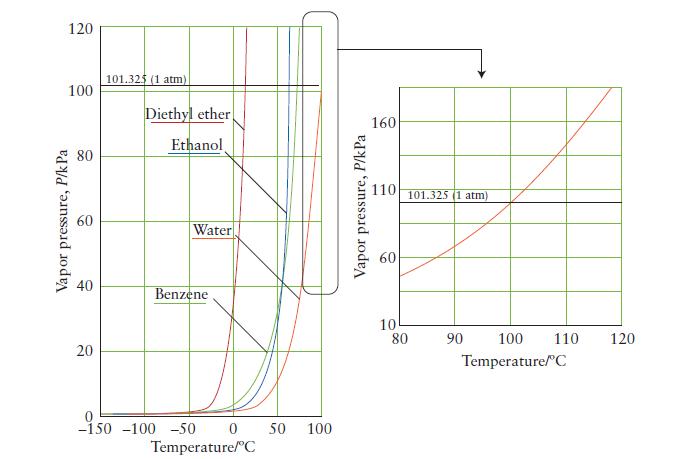
Transcribed Image Text:
120 100 Vapor pressure, P/k Pa 40 20 101.325 (1 atm) Diethyl ether Ethanol Water Benzene -1.50 -150 -100 -50 0 50 100 Temperature/C Vapor pressure, P/kPa 160 110 10 80 101.325 (1 atm) 90 100 110 Temperature/C 120
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The boiling point of a liquid is the temperature at which its vapor pressure eq...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The vapor pressure of benzene is 100.0 mmHg at 26.1C and 400.0 mmHg at 60.6C. What is the boiling point of benzene at 760.0 mmHg?
-
The boiling point of benzene is 80.1C. Estimate: (a) Its molar heat of vaporization and (b) Its vapor pressure at 74C.
-
A liquid mixture consisting of 100 kmol of 60 mol% benzene, 25 mol% toluene, and 15 mol% o-xylene is flashed at 1 atm and 100C. (a) Compute the amounts of liquid and vapor products and their...
-
In Exercises 7-9, find the indicated measure. The area of a circle is 380 square inches. Find the radius.
-
In field-flow fractionation, could a turbulent-flow field be used? Why or why not?
-
A certain output is manufactured at ksh.80 and sold at ksh.140 per unit. The product is such that if it is produced but not sold during a days time it becomes worthless. The daily sales records in...
-
Verify the algebra leading to Eq. (19.22) for radiation exchange between two plates facing each other. 1 21 -(Eb1 - Eb2) (19.22) 1/1 + 1/62-1
-
Crable and Tesch, partners in a systems consulting firm, budgeted the following professional labor hours for the year ended December 31, 2016: Partners . . . . . . . . . . . . . . . . . . . . . . . ....
-
The article "Marketing Myopia" by the late Harvard professor Theodore Levitt is considered by many to be one of the most influential articles on marketing strategy ever published. First published in...
-
Southeastern Foods has hired you to analyze their distribution-system design. The company has 11 distribution centers, with monthly volumes as listed below. Seven of these sites can support...
-
One step in the manufacture of sulfuric acid is the formation of sulfur trioxide by the combustion of SO 2 with O 2 in the presence of a vanadium(V) oxide catalyst. Suppose you are working out how to...
-
The phase diagram for carbon, shown here, indicates the extreme conditions that are needed to form diamonds from graphite. (a) At 2000 K, what is the minimum pressure needed before graphite changes...
-
When balancing reactions in Chapter 3, we did not mention that reactions must be charge balanced as well as mass balanced. What do charge balance and mass balance mean? How are redox reactions charge...
-
Prepare the Investment Objectives section of Patels IPS. Patel has been working with Zik for 10 years. At the beginning of the 10-year period, Zik forecasted that the equities in Patels portfolio...
-
Identify two strategies Delgado should use to earn a positive roll yield. Describe the specific steps needed to execute each strategy. Rosario Delgado is an investment manager in Spain. Delgados...
-
Calculate the net cash flow (in euros) to maintain the desired hedge. Show your calculations. With the US dollar currently trading at a forward premium and US interest rates lower than Spanish rates,...
-
Relative to Heydons existing fund, the new fund will most likely: A. hold a smaller number of stocks. B. rebalance at more regular intervals. C. see risk at the company level rather than the...
-
In a research program on human health risk from recreational contact with water contaminated with pathogenic microbiological material, the National Institute of Water and Atmospheric Research (NIWA)...
-
Set up a spreadsheet similar to Figure 12-11 to solve Problem 4. FIGURE 12-11 The housing contract from Problem 3 continues on into the next year, with the last housing start occurring in April, as...
-
During the month, services performed for customers on account amounted to $7,500 and collections from customers in payment of their accounts totaled $6,000. At the end of the month, the Accounts...
-
Provide an IUPAC name for each of the following compounds. a. b. c. d. e. I
-
Draw the structure of each of the following compounds. a. (R) -2-Ethoxy-1, 1-dimethylcyclobutane b. Cyclopropyl isopropyl ether
-
Show that the van der Waals and RedlichKwong equations of state reduce to the ideal gas law in the limit of low gas density.
-
Shan has informed you that she would like to cash out part of her portfolio in 8 years and use the proceeds to buy a vacation home in the south of France. Her portfolio has a current market value of...
-
Lloyd Enterprises has a project with the following cash flows: Year Cash Flow 0 -$200,000 1 50,000 2 100,000 3 150,000 4 40,000 5 ...
-
The FCFF and FCFE of OLE Co. last year were $17 billion and $13 billion, respectively. OLE's WACC is 11 percent, and its required rate of return for equity is 13 percent. FCFF is expected to grow at...

Study smarter with the SolutionInn App


