Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase:
Question:
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: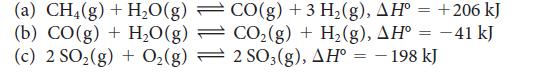
Transcribed Image Text:
(a) CH4(g) + H₂O(g) (b) CO(g) + H₂O(g) (c) 2 SO₂(g) + O₂(g) CO(g) +3 H₂(g), AH° = +206 kJ CO₂(g) + H₂(g), AH° = −41 kJ 2 SO3(g), AH° = - 198 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To predict the direction of shift for each equilibrium with a temperature increase we need to determ...View the full answer

Answered By

Anurag Agrawal
I am a highly enthusiastic person who likes to explain concepts in simplified language. Be it in my job role as a manager of 4 people or when I used to take classes for specially able kids at our university. I did this continuously for 3 years and my god, that was so fulfilling. Sometimes I've skipped my own classes just to teach these kids and help them get their fair share of opportunities, which they would have missed out on. This was the key driver for me during that time. But since I've joined my job I wasn't able to make time for my passion of teaching due to hectic schedules. But now I've made a commitment to teach for at least an hour a day.
I am highly proficient in school level math and science and reasonably good for college level. In addition to this I am especially interested in courses related to finance and economics. In quest to learn I recently gave the CFA level 1 in Dec 19, hopefully I'll clear it. Finger's crossed :)
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: 2 NO(g), AH = +57 kJ 2 X(g), where X is a halogen (a) NO4(g) (b) X(g) (c) Ni(s)...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Predict whether each of the following nuclides is stable or unstable (radioactive). If the nuclide is unstable, predict rhe type of radioactivity you would expect it to exhibit. a. 4519K b. 5626Fe c....
-
Use f(x) and g(x) to find a formula for each expression. Identify its domain. (a) (f + g)(x) (c) (fg)(x) (b) (f- g)(x) (d) (f/g)(x)
-
A wire of cross-sectional area A, length L 1 , resistivity 1 , and temperature coefficient 1 is connected end to end to a second wire of the same cross-sectional area, length L 2 , resistivity 2 ,...
-
When should a firm consider expanding from strictly domestic trade to international trade? When should it consider becoming further involved in international trade? What factors might affect the...
-
Stability analysis with heat transfer. Set up the equations for steady state for the Bnard problem. Now perturb the temperature and use an energy equation to derive an equation for the temperature...
-
An advocate of discounted cash flow analysis says, "Residual earnings valuation does not work well for companies like Coca-Cola, Cisco Systems, or Merck, which have substantial assets, like brands,...
-
- How can reading the garment label help you to become an informed shopper? Discuss why it is important to know the fibre content of a garment and why a consumer may want to know the country of...
-
As of December 31, Cookie Creations' year-end, the following adjusting entry data are provided. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. A count reveals that $45 of brochures and posters (supplies) were used....
-
Hexane, C 6 H 14 , and cyclohexane, C 6 H 12 , form an ideal solution. The vapor pressure of hexane is 151 Torr and that of cyclohexane is 98 Torr at 25.0C. Calculate the total vapor pressure of each...
-
A solution prepared by adding 0.50 g of a polymer to 0.200 L of toluene (methylbenzene, a common solvent) showed an osmotic pressure of 0.582 Torr at 20 8C. What is the molar mass of the polymer?
-
The stockholders equity section of Bellwood Brands 2021 balance sheet follows: During 2022, Bellwood completed the following transactions: November 9: Purchased 200 shares of its own stock to be...
-
Write up the following transactions in the books of J. Dunn: 2017 May 1 Started in business with cash 30,000. 2 Bought goods on time from T. Lamb 700. 3 Paid rent by cash 1,740. 4 Paid 25,000 of the...
-
Since monetary policy changes made through the fed funds rate occur with a lag, policymakers are usually more concerned with adjusting policy according to changes in the forecasted or expected...
-
These first three sections of the chapter have explained the meaning of a standard and the various different approaches to the creation of a standard. Read the sections again and satisfy yourself...
-
For the datapath from Figure 4.24, draw the logic diagram for the part of the control unit that implements just the first signal. Assume that we only need to support LW, SW, BEQ, ADD, and J (jump)...
-
Thumbtacks capital structure is shown in table below. If taxes are paid annually and Thumbtacks combined tax rate is 36 percent, determine the weighted average cost of capital. Loans Bonds Common...
-
If the loan in Problem 22 is paid off at the end of the thirtieth month (at the time of the 30th payment) what effect does this have on the effective annual interest rate? In Problem 22, the bank...
-
Using the parallel-axis theorem, determine the product of inertia of the area shown with respect to the centroidal x and y axes. 6 in. 9 in. 9 in- 4.5 in. in. 4.5 in.
-
The amino acid glycine dimerizes to form the dipeptide glycylglycine according to the reaction 2Glycine(s) Glycylglycine(s) + H 2 O(l) Calculate ÎS, ÎSsurroundings , and ÎSuniverse...
-
Draw the major product expected from each of the following reactions: (a) (b) ? Lindlar's catalyst Pt -? Ni,B -? Ni
-
Draw the major product expected when each of the following alkynes is treated with sodium in liquid ammonia: (a) (b) (c) (d)
-
Quilcene Oysteria farms and sells oysters in the Pacific Northwest. The company harvested and sold 7 , 4 0 0 pounds of oysters in August. The company s flexible budget for August appears below:...
-
How do hierarchical societies develop after some time, and which job do administration styles play in forming social standards and values inside an association ?
-
Mark Zuckerberg, is the owner of a well-known audio systems manufacturer. He wants to create a portfolio of suppliers for some of the electronics used in production. Based on historical data and...

Study smarter with the SolutionInn App


