Question: Suppose that 0.483 g of an unknown weak acid, HA, is dissolved in water. Titration of the solution with 0.250 m NaOH(aq) required 42.0 mL
Suppose that 0.483 g of an unknown weak acid, HA, is dissolved in water. Titration of the solution with 0.250 m NaOH(aq) required 42.0 mL to reach the stoichiometric point. After the addition of 21.0 mL, the pH of the solution was found to be 3.75.
(a) What is the molar mass of the acid?
(b) What is the value of pKa for the acid? Identify the acid in Table 6C.1.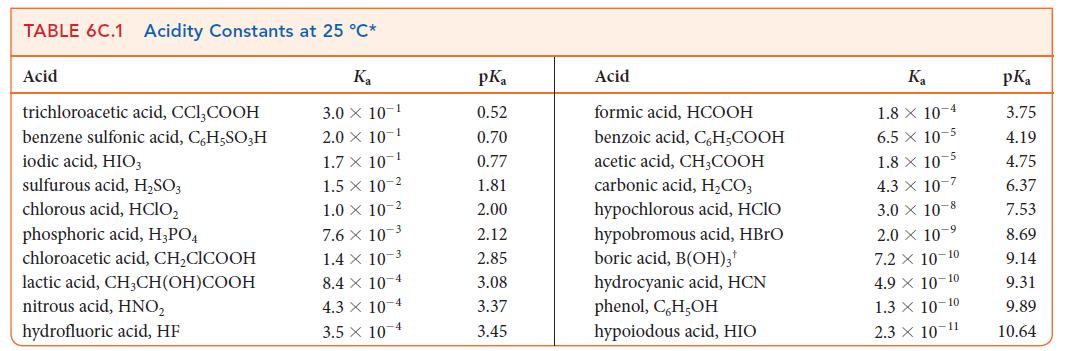
TABLE 6C.1 Acidity Constants at 25 C* Ka 3.0 X 10-1 2.0 X 10-1 1.7 X 10- Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C,H,SO3 H iodic acid, HIO3 sulfurous acid, HSO3 chlorous acid, HClO phosphoric acid, H3PO4 chloroacetic acid, CHClCOOH lactic acid, CH3CH(OH)COOH nitrous acid, HNO hydrofluoric acid, HF 1.5 x 10-2 1.0 10-2 7.6 x 10-3 1.4 x 10-3 8.4 x 10-4 4.3 x 10-4 3.5 x 10-4 pK 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C6HCOOH acetic acid, CH,COOH carbonic acid, HCO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3 hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO K 1.8 x 10-4 6.5 x 10-5 1.8 x 10-5 4.3 x 10-7 3.0 10-8 2.0 10- 7.2 x 10-10 4.9 10-10 1.3 10-10 2.3 10-11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Lets start by calculating the molar mass of the acid a Given Mass of HA 0483 g Volume of NaOH 420 mL ... View full answer

Get step-by-step solutions from verified subject matter experts


