Use Table 6H.2 to suggest suitable indicators for the titrations described in Exercises 6H.10 and 6H.12. Exercises
Question:
Use Table 6H.2 to suggest suitable indicators for the titrations described in Exercises 6H.10 and 6H.12.
Exercises 6H.10
Morphine, C17H19O3N, is a potent painkiller. Suppose you are studying morphine and need to predict the pH of a morphine solution during a titration. Calculate the pH at the stoichiometric point of the titration of 30.00 mL of 0.0172 m C17H19O3N(aq) with 0.0160 m HCl(aq).
Exercises 6H.12
Suppose that 0.483 g of an unknown weak acid, HA, is dissolved in water. Titration of the solution with 0.250 m NaOH(aq) required 42.0 mL to reach the stoichiometric point. After the addition of 21.0 mL, the pH of the solution was found to be 3.75.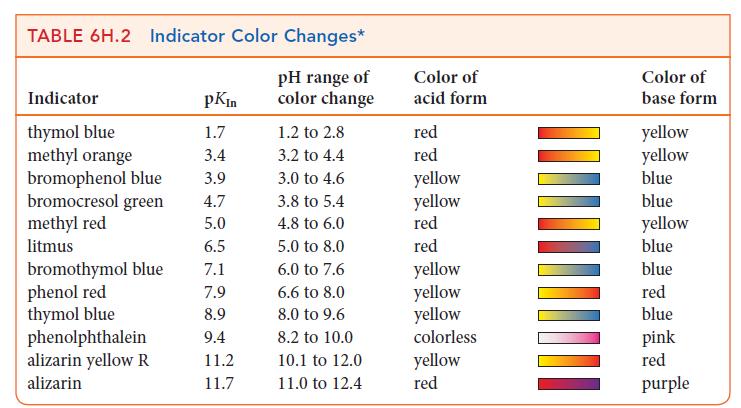
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





