Suppose that 50.0 mL of 0.25 m CH 3 NH 2 (aq) is titrated with 0.35 m
Question:
Suppose that 50.0 mL of 0.25 m CH3NH2(aq) is titrated with 0.35 m HCl(aq).
(a) What is the initial pH of the 0.25 m CH3NH2(aq)?
(b) What is the pH after the addition of 15.0 mL of 0.35 m HCl(aq)?
(c) What volume of 0.35 m HCl(aq) is required to reach half way to the stoichiometric point?
(d) Calculate the pH at the halfway point.
(e) What volume of 0.35 m HCl(aq) is required to reach the stoichiometric point?
(f) Calculate the pH at the stoichiometric point.
(g) Use Table 6H.2 to select an indicator for the titration.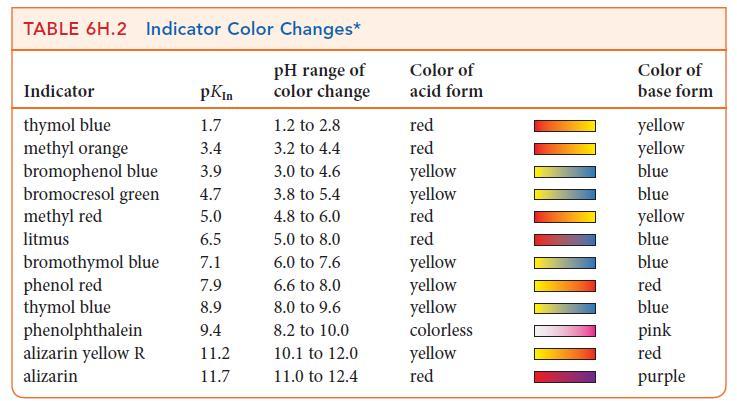
Transcribed Image Text:
TABLE 6H.2 Indicator Color Changes* pH range of color change Indicator thymol blue methyl orange pKin 1.7 3.4 bromophenol blue 3.9 4.7 bromocresol green methyl red 5.0 litmus 6.5 7.1 7.9 8.9 9.4 11.2 11.7 bromothymol blue phenol red thymol blue phenolphthalein alizarin yellow R alizarin 1.2 to 2.8 3.2 to 4.4 3.0 to 4.6 3.8 to 5.4 4.8 to 6.0 5.0 to 8.0 6.0 to 7.6 6.6 to 8.0 8.0 to 9.6 8.2 to 10.0 10.1 to 12.0 11.0 to 12.4 Color of acid form red red yellow yellow red red yellow yellow yellow colorless yellow red Color of base form yellow yellow blue blue yellow blue blue red blue pink red purple
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a Initial pH of 025 M CH3NH2 CH3NH2aq H2O1CH3NH3 aq OHaq K 36 10 CH3NH3 OH CH3NH2 Concentration mol ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Suppose that 15.0 mL of 0.15 m NH 3 (aq) is titrated with 0.10 m HCl(aq). (a) What is the initial pH of the 0.15 m NH 3 (aq)? (b) What is the pH after the addition of 15.0 mL of 0.10 m HCl(aq)? (c)...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A set of 2M biorthogonal signals is obtained from a set of M orthogonal signals by augmenting it with the negative of each signal in the set. (a) The extension of orthogonal to biorthogonal signals...
-
A wave with frequency of 1200 Hz propagates along a wire that is under a tension of 800 N. The wavelength of the wave is 24 cm. What will be the wavelength if the tension is decreased to 600 N and...
-
1. Make a list of the elements that are interrelated or interdependent. Then write a paragraph stating why it is critical to monitor these elements closely. 2. Decide on the boundaries and ultimate...
-
The finalists can be viewed at http://www .sciencemag.org/projects/data-stories/finalists. Pick a video that interests you, watch it, and answer the following questions: (a) Give a link to the chosen...
-
For each definition (or portion of a definition) in the first column, select the term that most closely applies. Each term may be used only once or not at all. Definition (or Portion) a. A detailed...
-
If I invest a single amount of $14,000 in an account earning 8% p.a. compounding quarterly for 5 years, how much interest will I have earned in those 5 years?
-
White Ski Resorts operates a series of ski resorts in northern Europe and reports under IFRS. On June 30, 20X0, White purchased land for 3,000,000. White reports land values on the balance sheet...
-
Which is the stronger acid, hydrocyanic acid, HCN, or the ammonium ion, NH 4 ? Justify your answer.
-
Zinc(II) readily forms the complex ion Zn(OH) 4 2 . Explain how this fact can be used to distinguish a solution of ZnCl 2 from MgCl 2 .
-
The differential equation of a spring/mass system is x'' + 16x = 0. If the mass is initially released from a point 1 meter above the equilibrium position with a downward velocity of 3 m/s, the...
-
(a) Write an expression for the kinetic energy of an object in terms of its momentum \(p\) and inertia \(m\). (b) If kinetic energy can be written in terms of momentum, how can the kinetic energy of...
-
Explain what is going on in terms of energy when your brakes overheat as you use them continuously coasting down a steep hill on a bike or in a car.
-
An assembled system consists of cart A of inertia \(m_{A}\), cart B of inertia \(m_{\mathrm{B}}\), and a spring of negligible inertia, clamped together so that the fully compressed spring is aligned...
-
A mysterious crate has shown up at your place of work, Firecracker Company, and you are told to measure its inertia. It is too heavy to lift, but it rolls smoothly on casters. Getting an inspiration,...
-
Two carts are initially moving to the right on a low-friction track, with cart 1 behind cart 2 . Cart 1 has a speed twice that of cart 2 and so moves up and rear-ends cart 2 , which has twice the...
-
a. Use the data given to test the following hypotheses. H0: = 1200 Ha: > 1200 = 1215, n = 113, = 100, = .10 b. Use the p-value to obtain the results. c. Solve for the critical value required to...
-
Wilsons Auto Repair ended 2011 with Accounts Receivable of $85,000 and a credit balance in Allowance for Uncollectible Accounts balance of $11,000. During 2012, Wilsons Auto Repair had the following...
-
Amphotericin B is a powerful antifungal agent used for intravenous treatment of severe fungal infections. Identify the most acidic proton in this compound: , Amphotericin B NH2
-
Predict the position of equilibrium for each of the following reactions: (a) (b) (c) HO NH NH2
-
As we will learn in Chapter 21, treating a lactone (a cyclic ester) with sodium hydroxide will initially produce an anion: This anion rapidly undergoes an intramolecular proton transfer, in which the...
-
Write a 5 -8 paragraph essay in MLA styles about "Drones" with references. In order to generate MLA citations, use CCC library, Purdue Owl; citethisforme.com: this is a citation generator for MLA and...
-
3. McClain, Edwards, Shiver, and Smith (MESS) LLC is considering the purchase of new automated cleaning equipment. The industrial engineer for the company, David "the Dirtman" R., has been asked to...
-
Suppose that V is a four-dimensional vector space with basis V1, V2, V3, V4. Let T: VR be a linear transformation such that T(VI) = T(V2) = = T(V3) T(V4)= (5, -6, -9) (-1,-1,-9) (6, -3, 5) (-9,9,5)...

Study smarter with the SolutionInn App


