The following data were collected for a gas sample consisting of 1.00 mol of molecules in a
Question:
The following data were collected for a gas sample consisting of 1.00 mol of molecules in a rigid container.
(a) Determine the volume of the sample.
(b) Plot the data either by hand or using a spreadsheet. Now suppose you add an additional 1.00 mol of gas molecules into the same volume. Plot the corresponding line for the second sample.
(c) At what temperature (in kelvins) do the two lines intersect?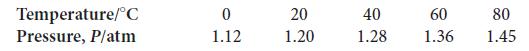
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





