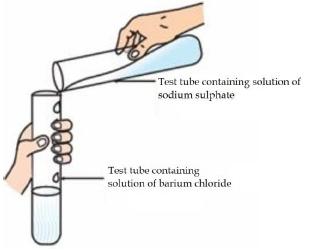
Question: 1. Identify the product which represents the solid state in the above reaction. a) Barium chloride b) Barium sulphate c) Sodium chloride d) Sodium sulphate
1. Identify the product which represents the solid state in the above reaction.
 a) Barium chloride
a) Barium chloride
b) Barium sulphate
c) Sodium chloride
d) Sodium sulphate
2. The colour of the solution observed after 30 minutes of placing zinc metal to copper sulphate solution is
a) Blue
b) Colourless
c) Dirty green
d) Reddish Brown
3. Mild non-corrosive basic salt is
a) Ca (OH)2
b) NaCl
c) NaOH
d) NaHCO3
4. On adding dilute sulphuric acid to a test tube containing a metal ‘X’, a colourless gas is produced when a burning match stick is brought near it. Which of the following correctly
represents metal ‘X’?
a) Sodium
b) Sulphur
c) Copper
d) Silver
Test tube containing solution of sodium sulphate Test tube containing solution of barium chloride
Step by Step Solution
There are 3 Steps involved in it
1 b Barium Sulphate Barium chloride and Sodium Sulphate reacts to form Barium Sulphate and S... View full answer

Get step-by-step solutions from verified subject matter experts


