Question: The reaction [mathrm{I}^{-}(a q)+mathrm{OCl}^{-}(a q) longrightarrow mathrm{IO}^{-}(a q)+mathrm{Cl}^{-}(a q)] is believed to occur by the following mechanism: Write the rate law for this reaction. Since
The reaction
\[\mathrm{I}^{-}(a q)+\mathrm{OCl}^{-}(a q) \longrightarrow \mathrm{IO}^{-}(a q)+\mathrm{Cl}^{-}(a q)\]
is believed to occur by the following mechanism:
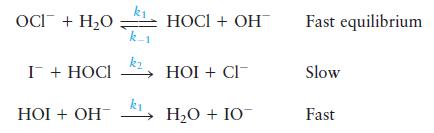
Write the rate law for this reaction. Since the reaction is in aqueous solution, the effective concentration of water remains constant. Thus the rate of the forward reaction in the first step can be written as
\[\text { Rate }=k\left[\mathrm{H}_{2} \mathrm{O}ight]\left[\mathrm{OCl}^{-}ight]=k_{1}\left[\mathrm{OCl}^{-}ight]\]
OCI + HO I + HOCI 2 HOI + CI- k HOI + OH HOCI + OH HO + IO Fast equilibrium Slow Fast
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
To write the rate law for the reaction we need to consider the slowest step in the mechanism ... View full answer

Get step-by-step solutions from verified subject matter experts


