The solubility of benzoic acid, is (0.34 mathrm{~g} / 100 mathrm{~mL}) in water at (25^{circ} mathrm{C}) and
Question:
The solubility of benzoic acid,
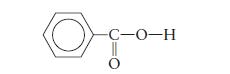
is \(0.34 \mathrm{~g} / 100 \mathrm{~mL}\) in water at \(25^{\circ} \mathrm{C}\) and \(10.0 \mathrm{~g} / 100 \mathrm{~mL}\) in benzene \(\left(\mathrm{C}_{6} \mathrm{H}_{6}ight)\) at \(25^{\circ} \mathrm{C}\). Rationalize this solubility behavior. For a \(1.0 \mathrm{~m}\) solution of benzoic acid in benzene, would the measured freezing-point depression be equal to, greater than, or less than \(5.12^{\circ} \mathrm{C}\) ? \(\left(K_{\mathrm{f}}=5.12^{\circ} \mathrm{C} \mathrm{kg} / \mathrm{mol}ight.\) for benzene.)
Transcribed Image Text:
-C-0-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To rationalize the solubility behavior of benzoic acid in water versus benzene we can turn to the pr...View the full answer

Answered By

Nicholas Maina
Throughout my tutoring journey, I've amassed a wealth of hands-on experience and honed a diverse set of skills that enable me to guide students towards mastering complex subjects. My proficiency as a tutor rests on several key pillars:
1. Subject Mastery:
With a comprehensive understanding of a wide range of subjects spanning mathematics, science, humanities, and more, I can adeptly explain intricate concepts and break them down into digestible chunks. My proficiency extends to offering real-world applications, ensuring students grasp the practical relevance of their studies.
2. Individualized Guidance:
Recognizing that every student learns differently, I tailor my approach to accommodate various learning styles and paces. Through personalized interactions, I identify a student's strengths and areas for improvement, allowing me to craft targeted lessons that foster a deeper understanding of the material.
3. Problem-Solving Facilitation:
I excel in guiding students through problem-solving processes and encouraging critical thinking and analytical skills. By walking learners through step-by-step solutions and addressing their questions in a coherent manner, I empower them to approach challenges with confidence.
4. Effective Communication:
My tutoring proficiency is founded on clear and concise communication. I have the ability to convey complex ideas in an accessible manner, fostering a strong student-tutor rapport that encourages open dialogue and fruitful discussions.
5. Adaptability and Patience:
Tutoring is a dynamic process, and I have cultivated adaptability and patience to cater to evolving learning needs. I remain patient through difficulties, adjusting my teaching methods as necessary to ensure that students overcome obstacles and achieve their goals.
6. Interactive Learning:
Interactive learning lies at the heart of my approach. By engaging students in discussions, brainstorming sessions, and interactive exercises, I foster a stimulating learning environment that encourages active participation and long-term retention.
7. Continuous Improvement:
My dedication to being an effective tutor is a journey of continuous improvement. I regularly seek feedback and stay updated on educational methodologies, integrating new insights to refine my tutoring techniques and provide an even more enriching learning experience.
In essence, my hands-on experience as a tutor equips me with the tools to facilitate comprehensive understanding, critical thinking, and academic success. I am committed to helping students realize their full potential and fostering a passion for lifelong learning.
4.90+
5+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
In Exercises 51-54, a. determine the value that the function f approaches as the magnitude of x increases. Is f (x) greater than or less than this value when b. x is positive and large in magnitude...
-
The accompanying data are x = cost (cents per serving) and y = fiber content (grams per serving) for 18 high-fiber cereals rated by Consumer Reports (www .consumerreports.org/health). a. Compute and...
-
Which statement is correct? A) Tax credits reduce tax liability on a dollar-for-dollarbasis. B) Tax deductions reduce tax liability on a dollar-for-dollarbasis. C) The benefit of a tax credit depends...
-
The population of the world was about 5.3 billion in 1990. Birth rates in the 1990s ranged from 35 to 40 million per year and death rates ranged from 15 to 20 million per year. Lets assume that the...
-
If sunlight can be conceived of as a beam of photons, each of which carries a certain amount of energy and momentum, why dont we experience (or feel) any recoil as these particles collide with our...
-
True or False: If \(P W>0\), then \(I R R>M A R R\).
-
Fallow Co. had the following transactions during the current period. Mar. 2 Issued 5,000 shares of $1 par value common stock to attorneys in payment of a bill for $38,000 for services provided in...
-
= 5. Find the path delay D of the logic chain shown below. Assume Cout 15C, and the numbers on the gate indicates the input capacitance Cin- In 5C 5C (2-points) 3C 4C 5C 4C Out Cout
-
A sample containing \(0.0500 \mathrm{~mol}\) of \(\mathrm{Fe}_{2}\left(\mathrm{SO}_{4}ight)_{3}\) is dissolved in enough water to make \(1.00 \mathrm{~L}\) of solution. This solution contains...
-
The Tyndall effect is often used to distinguish between a colloidal suspension and a true solution. Explain.
-
The following is a summary of information presented on the financial statements of a company on December 31, 2017. With respect to net sales revenue, a horizontal analysis reveals ________. A....
-
If your country has competition or antitrust laws, find a landmark case, and explain whether you support the plaintiff or the defendant. If your country does not have competition or antitrust laws,...
-
An advertisement for a brand-name camera stated that the cameras are inspected and that 60 % are rejected for the slightest imperfections. To test this assertion, you observe the inspection of a...
-
As a staff member at one of the 48 state attorney generals offices that are investigating Google, you need to write a report recommending to the attorney general of your state what actions to takeand...
-
Assuming you work for a New Zealand company exporting a container of kiwi fruit to Haiti. The customs official informs you that there is a delay in clearing your container and it may last a month....
-
As a CEO leading an acquisition of a famous foreign firm, you are interviewed by a reporter from the host country. The reporter asks, A lot of people in our country are mad about this foreign...
-
Stockholders of the company converted 10,000 shares of $50 par preferred stock into 50,000 shares of $1 par common stock. The preferred shares were originally issued for $53 per share. Make the...
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
The production of steel from iron ore is endothermic. To reduce the heat that must be supplied, engineers need to find the lowest temperature at which the desired reactions are spontaneous. Estimate...
-
By assuming that the lattice enthalpy of NaCl 2 is the same as that of MgCl 2 , use enthalpy arguments based on data in Appendix 2A, explain why NaCl 2 is an unlikely compound. 2A THERMODYNAMIC DATA...
-
The standard entropy of vaporization of acetone is approximately 85 J K 1 mol 1 at its boiling point. (a) Estimate the standard enthalpy of vaporization of acetone at its boiling point of 56.2 C....
-
es Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement for a recent month for the two games appears below: Sales Variable expenses Claimjumper...
-
Thermal Rising, Incorporated, makes paragliders for sale through specialty sporting goods stores. The company has a standard paraglider model, but also makes custom-designed paragliders. Management...
-
Franklin Corporation, which has three divisions, is preparing its sales budget. Each division expects a different growth rate because economic conditions vary in different regions of the country. The...

Study smarter with the SolutionInn App


