The temperature-dependence of the vapor pressure of phosphoryl chloride difluoride (OPClF 2 ) has been measured: (a)
Question:
The temperature-dependence of the vapor pressure of phosphoryl chloride difluoride (OPClF2) has been measured: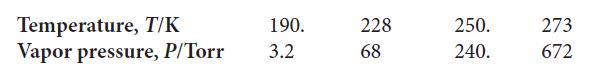
(a) Plot ln P against 1/T (this plot is best created with the aid of a computer or a graphing calculator that can calculate a linear least squares fit to the data).
(b) From the plot (or a linear equation derived from it) in part (a), determine the standard enthalpy of vaporization of OPClF2;
(c) the standard entropy of vaporization of OPClF2; and
(d) the normal boiling point of OPClF2.
(e) If the pressure of a sample of OPClF2 is reduced to 15 Torr, at what temperature will the sample boil?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





