Question: The wavefunction for a particle in a one- dimensional box is given in Eq. 2. Confirm that the probability of finding the particle in the
The wavefunction for a particle in a one- dimensional box is given in Eq. 2. Confirm that the probability of finding the particle in the left half of the box is 1/2 regardless of the value of n.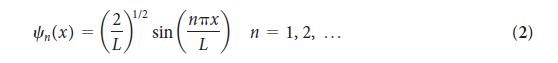
1/2 n(x) = (*) - () * sin(x) n = 1, 2, ... (2)
Step by Step Solution
3.44 Rating (163 Votes )
There are 3 Steps involved in it
Integrate over the left half of t... View full answer

Get step-by-step solutions from verified subject matter experts


