Use data in Table 4C.1 to calculate the entropy change for (a) The vaporization of 2.40 mol
Question:
Use data in Table 4C.1 to calculate the entropy change for
(a) The vaporization of 2.40 mol H2O(l) at 100. °C and 1 atm;
(b) The freezing of 4.50 g of ethanol, C2H5OH, at 158.7 K.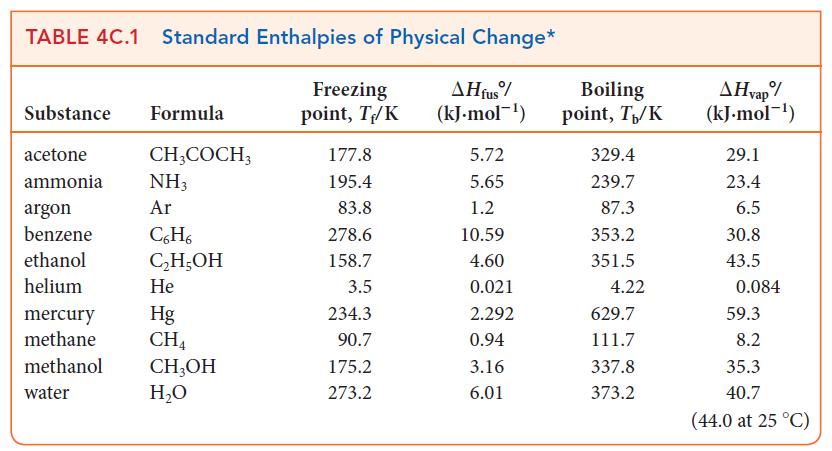
Transcribed Image Text:
TABLE 4C.1 Standard Enthalpies of Physical Change* Freezing point, T/K Substance Formula acetone ammonia argon benzene ethanol helium CH3COCH3 NH3 Ar C6H6 C₂H5OH He mercury Hg methane CH4 methanol CH3OH water H₂O 177.8 195.4 83.8 278.6 158.7 3.5 234.3 90.7 175.2 273.2 AH fus (kJ.mol-¹) 5.72 5.65 1.2 10.59 4.60 0.021 2.292 0.94 3.16 6.01 Boiling point, T₁/K 329.4 239.7 87.3 353.2 351.5 4.22 629.7 111.7 337.8 373.2 A Hvap%/ (kJ.mol-¹) 29.1 23.4 6.5 30.8 43.5 0.084 59.3 8.2 35.3 40.7 (44.0 at 25 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The entropy change AS for a process is given by the formula AS AH where AH is the enthalpy ...View the full answer

Answered By

Raunak Agarwal
Teaching is my hobby and now my profession. I teach students of CA and CFA(USA) in batches of 100 students and have a 5 year experience.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the entropy change for the vaporization of liquid methane and hexane using the following data: Compare the molar volume of gaseous methane at 112 K with that of gaseous hexane at 342 K. How...
-
Calculate the entropy change for a process in which 3.00 moles of liquid water at 0oC is mixed with 1.00 mole of water at 100.oC in a perfectly insulated container. (Assume that the molar heat...
-
Use data in Table 4C.1 or Appendix 2A to calculate the entropy change for (a) The freezing of 1.00 mol H 2 O(l) at 0.00C; (b) The vaporization of 50.0 g of ethanol, C 2 H 5 OH, at 351.5 K. TABLE 4C.1...
-
CMOS Chips is hedging a 20-year, $21 million, 8% bond payable with a 20-year interest rate swap and has designated the swap as a fair value hedge. The agreement called for CMOS to receive payment...
-
(a) Describe the philosophy and approach of just-in-time processing. (b) Identify the major elements of JIT processing.
-
Assuming the vectors in Fig. 9.42 are in equilibrium, find T 1 and T 2 . T Fig. 9.42 255 N 58.3 37.2 T
-
A \(2000-\mathrm{kg}\) car burns gasoline with \(25 \%\) efficiency. Accelerating from rest, how fast would this car go upon burning \(0.040 \mathrm{~L}\) of gasoline if \(1.0 \mathrm{~L}\) of...
-
Prepare a cost of goods sold budget for the Highlands Manufacturing Co. for the year ended December 31, 2013, from the following estimates. Inventories of production units: Direct materials purchased...
-
Duncan's Diamond Bit Drilling Corporation (Duncan) purchased the following assets in 2023. Assume its taxable income was $60,000 for purposes of computing the 179 expense deduction. Asset Purchase...
-
A sample of gas in a cylinder of volume 3.42 L at 298 K and 2.57 atm expands to 7.39 L by two different pathways. Path A is an isothermal, reversible expansion. Path B has two steps. In the first...
-
In the manufacture of nitric acid by the oxidation of ammonia, the first product is nitric oxide, which is then oxidized to nitrogen dioxide. From the standard reaction enthalpies, calculate the...
-
On April 1, Paine Co. began construction of a small building. Payments of $180,000 were made monthly for four months beginning on April 1. The building was completed and ready for occupancy on August...
-
A bailee must exercise reasonable care to preserve the bailed property. (True/False)
-
A license is a revocable right of a person to come onto another persons land. (True/False)
-
A fee simple absolute is potentially infinite in duration and can be disposed of by deed or by will. (True/False)
-
National Stores, Inc., decides to file for bankruptcy. Under which chapter of the Bankruptcy Code can a corporation file a petition for bankruptcy? a. Chapter 7 only. b. Chapter 11 only. c. Chapter...
-
Who owns property after an accession?
-
Could the burdens of a regulation be either progressive or regressive, like the effects of a tax?
-
What is taxable income, and what is the formula for determining taxable income?
-
Another way of producing highly crosslinked polyesters is to use glycerol. Alkyd resins are a polymer of this type. The polymer forms very tough coatings when baked onto a surface and is used in...
-
Distinguish between the primary, secondary, and tertian structures of a protein. Give examples of the types of forces that maintain each ty pe of structure.
-
Which of the amino acids in Fig. contain the following functional groups in their R group? a. Alcohol b. Carboxylic acid c. Amine d. Amide Nonpolar R groups OH OH HyC Glycine (Gly) Alanine Ala) OH ...
-
Multiply. 7 12 20 49 7 12 20 49 = (Type an integer or a simplified fraction.)
-
What is required in order to establish a team-based culture and structure?
-
Compute the standard deviation. The formula for computing the standard deviation is given by 8 = n (; - )2 i=1 n-1 The process for computing the standard deviation can also be expressed in words...

Study smarter with the SolutionInn App


