In the manufacture of nitric acid by the oxidation of ammonia, the first product is nitric oxide,
Question:
In the manufacture of nitric acid by the oxidation of ammonia, the first product is nitric oxide, which is then oxidized to nitrogen dioxide. From the standard reaction enthalpies,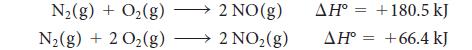
calculate the standard reaction enthalpy for the oxidation of nitric oxide to nitrogen dioxide:![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





