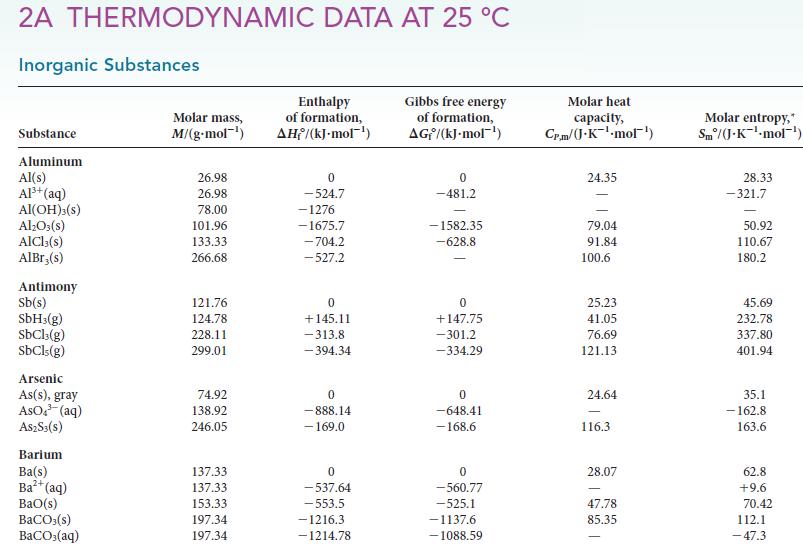
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of
Question:
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions:
(a) The removal of hydrogen sulfide from natural gas:![]()
(b) The oxidation of ammonia:![]()
(c) The formation of phosphorous acid:![]()
Transcribed Image Text:
2 HS(g) + SO (g) - 3 S(s) + 2 HO (1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a AH3AHSs2AHHO12AHHSgAHSOg 3 mol0 kJ mol2 mol28583 kJ mol 2 m...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions: (a) The final stage in the production of nitric acid: (b) The...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
The information in Table 4D.2 must be determined from experimental data, but because some reactions cannot be carried out directly, chemists who compile these types of tables commonly use enthalpies...
-
47GP: Chapter: CH0 CH1 CH2 CH3 CH4 CH5 CH6 CH7 CH8 CH9 CH10 CH11 CH12 CH13 CH14 CH15 CH16 CH17 CH18 CH19 CH20 CH21 CH22 CH23 CH24 CH25 CH26 CH27 CH28 CH29 CH30 Problem: 1CQ 1MCP 1P 2CQ 2MCP 2P 3CQ...
-
In Montego Company, total material costs are $32,000, and total conversion costs are $54,000. Equivalent units of production are materials 10,000 and conversion costs 12,000. Compute the unit costs...
-
Solve the triangles with the given parts. a = 89.45, c = 37.36, C = 15.62
-
A 60-g Mars bar will supply you with \(1095 \mathrm{~kJ}\) of energy. How many of them would you need to get enough energy to climb the first \(800 \mathrm{~m}\) of the highest structure in the...
-
Refer to Exercise 20-18. Fiber Systems needs 84,000 optical switches. By outsourcing them, Fiber Systems can use its idle facilities to manufacture another product that will contribute $253,000 to...
-
You can find this question from Excel worksheet "poison pill question". Twilight has 1,000,000 shares outstanding, and the current stock price is $25 per share. Yesterday, Activist Apollo made a...
-
Calculate the standard enthalpy of formation of dinitrogen pentoxide from the following data: and from the standard enthalpy of formation of nitric oxide, NO. 2 NO(g) + O2(g) + O2(g) 4 NO2(g) 2...
-
Calculate the reaction enthalpy for the formation of anhydrous aluminum bromide, 2 Al(s) + 3 Br 2 (l) 2 AlBr 3 (s), from the following data: 2 Al(s) + 6 HBr(aq) - HBr(g) H(g) + Br (1) AlBr3 (s) 2...
-
Consider the following query: Which of the following statements is not correct? a. The query retrieves the product number, product name, and available quantity of each product thanks to the left...
-
Technological developments deskill the global workforce. For example, factory-built, flat-pack furniture cut out the role of experienced carpenters. Similarly, with some vehicles having on-board...
-
In 2013, a clothing factory in Bangladesh collapsed, killing 1,138 people. Some 27 global brands, including Walmart and Benetton, were using the factory. One year on, these two corporations were...
-
Scott Emmons was working for Neiman Marcus, the luxury retailer, as an enterprise architect when he realized a big gap in how their stores were handling technology. Customers were showing up with...
-
While important, rules may sometimes create more problems than they resolve. Websites like TripAdvisor are valuable for tourism, providing businesses with greater exposure. The problem is not all...
-
An unexpected ethical issue arose when Hungary, Romania, and Bulgaria joined the European Union (EU). The start of the free movement of workers across the EU meant that workers from these countries...
-
Why do you think news reporters are more informed than average citizens about public policy issues?
-
At Glass Company, materials are added at the beginning of the process and conversion costs are added uniformly. Work in process, beginning: Number of units Transferred - in costs Direct materials...
-
Alkanes and aromatics are fairly stable compounds. To make them react, a special catalyst must be present. What catalyst must be present when reacting CI2 with an alkane or with benzene? Adding CI2...
-
The following are some other organic reactions covered in Section 21.4. Give an example to illustrate each type of reaction. a. Adding H2O to an alkene (in the presence of H+) yields an alcohol. b....
-
In the presence of light, chlorine can substitute for one (or more) of the hydrogens in an alkane. For the following reactions, draw the possible monochlorination products. hr 2,2-dimethylpropane Cl2...
-
What would you observe in procedural step 2 under Flow chart tests if 6 M were used in place of 6 M ?
-
What principles of financial management , might have made a difference to the NYC Opera during its approach to bankruptcy?
-
The hospitality industry had to re - evaluate several of its operational strategies due to the pandemic, even with "the new normal" the industry has changed. For this assignment, you will evaluate...

Study smarter with the SolutionInn App


