Question: Write balanced half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions: (a) Pb(NO3)2(aq) + KSO4(aq) PbSO4(s) +
Write balanced half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions: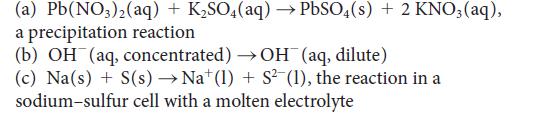
(a) Pb(NO3)2(aq) + KSO4(aq) PbSO4(s) + 2 KNO3(aq), a precipitation reaction (b) OH(aq, concentrated) OH(aq, dilute) (c) Na(s) + S(s) Nat (1) + S(1), the reaction in a sodium-sulfur cell with a molten electrolyte
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
a PbNO32aq K2SO4aq PbSOs 2 KNO3aq This is a precipitation reaction The halfreactions involved are th... View full answer

Get step-by-step solutions from verified subject matter experts


