Question: Write the half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions: (a) AgBr(s) Ag (aq) + Br
Write the half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions: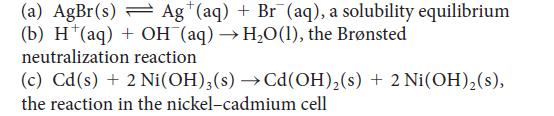
(a) AgBr(s) Ag (aq) + Br (aq), a solubility equilibrium (b) H(aq) + OH (aq) HO(1), the Brnsted neutralization reaction (c) Cd(s) + 2 Ni(OH)3(s) Cd(OH) (s)+ 2 Ni(OH) (s), the reaction in the nickel-cadmium cell
Step by Step Solution
3.34 Rating (163 Votes )
There are 3 Steps involved in it
a anode Ags Braq AgBrs e cathode Agtaq e Ags AgsA... View full answer

Get step-by-step solutions from verified subject matter experts


