Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic
Question:
Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic cells: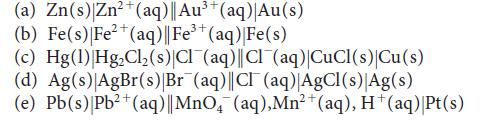
Transcribed Image Text:
2+ (a) Zn(s) Zn²+ (aq)|| Au³+ (aq)| Au(s) 3+ (b) Fe(s) | Fe²+ (aq) || Fe³+ (aq) |Fe(s) (c) Hg(1) Hg₂Cl₂ (s)|Cl(aq)||Cl(aq)|CuCl(s) Cu(s) (d) Ag(s)|AgBr(s)|Br (aq)||CI (aq)|AgCl(s)|Ag(s) (e) Pb(s) |Pb²+ (aq) || MnO₂ (aq), Mn²+ (aq), H+ (aq)|Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a Zns Zn2aq Au3aq Aus Halfreactions Anode Zns Zn2aq 2e oxidation Cathode Au3aq 3e Au...View the full answer

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Explain how the following Arduino codes affect the motion of the motor. Which direction is the motor moving? Why the map commands are required? (6%) duty1 = 40; duty2 = 60; dutyl-map...
-
Write the half reactions for the electrolysis of the elements listed in Exercise 3.
-
Write a balanced equation for each of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of...
-
If A = -2 6 1 -7 1 then det (A) = an and A-1 =
-
Scrap at time of sale or at time of production, journal entries (continuation of 18-35). Assume that Job #10 of Whitefish Machine Shop generates normal scrap with a total sales value of $300 (it is...
-
The Arnold Palmer Hospital (APH) in Orlando, Florida, is one of the busiest and most respected hospitals for the medical treatment of children and women in the U.S. Since its opening on golfing...
-
What is the difference between null and alternative hypothesis?
-
Selected data on merchandise inventory, purchases, and sales for Jaffe Co. and Coronado Co. are as follows: Instructions 1. Determine the estimated cost of the merchandise inventory of Jaffe Co. on...
-
Previously we addressed the problem of a pinned-joint truss in the shape of a regular pentagon that is supported on rollers and loaded by a single vertical force F. We want to determine the loads in...
-
The molar solubility of silver sulfite, Ag 2 SO 3 , is 1.55 * 10 5 mol L 1 . What is the K sp of silver sulfite?
-
Silver emulsion photographic film is now largely obsolete for amateur photography, but it is still used in a variety of medical and technical applications. You are working on the improvement of a...
-
Develop a requirements description for an interesting device. The device may be a household appliance, a computer peripheral, or whatever you wish.
-
We wish to prove the following statement: For all integers a and b, if 5 divides a or 5 divides b, then 5 divides (5a + b). In this case, it is to attempt to find an example. In this case, it is to...
-
b Employer identification number (EIN) 26-3356489 c Employer's name, address, and ZIP code General Motors LLC 9832 Detroit Way Detroit, MI 54569 d Control number e Employee's first name and initial...
-
Mr Apricot Angelfish is an Australian, born in Melbourne. Apricot is a one of the top Michelin Star chefs in the world and has been working in Hong Kong for 20 years. He is a permanent resident in...
-
Home work Differential amplifier with Darlington input transistors: A) If Rb-0 prove that: Rid=4(hfe+1)hie1=4(hfe+1)hib1 B) If Rb+ 0 prove that: CMRR Ro3 Rb (1+hfe) 2hib1+ VI Rb where Ro3 = r03...
-
A parent company acquired 80% of the stock of a subsidiary company on January 1, 2015. The total fair value of the controlling interest and the noncontrolling interest on that date was 94,000 in...
-
Suppose a factory accumulates costs in five separate cost pools. Would the equivalent units be different for each of these cost pools?
-
Annual dividends of ATTA Corp grew from $0.96 in 2005 to $1.76 in 2017. What was the annual growth rate?
-
Consider the couple Ox + e Red with the oxidized and redu ced species at unit activity. What must be the value of E for this half-cell if the reductant Red is to liberate hydrogen at 1 atm from a....
-
By finding appropriate half-cell reactions, calculate the equilibrium constant at 298.15 K for the following reactions: a. 4NiOOH(s) + 2 2 O(l) 4Ni(OH) 2 (s) + O 2 (g) b. 4NO 3 (aq)+ 4H + (aq)...
-
The cell potential E for the cell Pt(s)|H 2 (g, a H2 = 1) H + (aq, a H+ = 1)NaCl(aq, m = 0.300) AgCl(s) Ag(s) is +0.260 V. Determine Cl assuming that = Na+ = Cl .
-
MOTOROLA, INC., AND SUBSIDIARIES* CONSOLIDATED STATEMENTS OF OPERATIONS (IN PART) Years Ended December 31, (In millions, except per share amounts) 2008 2007 2006 Other Charges 3. Other Financial Data...
-
Required Information [The following information applies to the questions displayed below.] Vitamix reports the following information for its year ended December 31: Cash sales Sales on credit General...
-
You are required to collect annual reports of the selected two companies for the financial years ending on June 30, 2021 and 2022. 1. Identify and list the relevant IASS/IFRSS related to current and...

Study smarter with the SolutionInn App


